Abstract
Stem cells generate neurons in discrete regions in the postnatal mammalian brain. However, the extent of neurogenesis in the adult human brain has been difficult to establish. We have taken advantage of the integration of 14C, generated by nuclear bomb tests during the Cold War, in DNA to establish the age of neurons in the major areas of the human cerebral neocortex. Together with the analysis of the neocortex from patients who received BrdU, which integrates in the DNA of dividing cells, our results demonstrate that, whereas nonneuronal cells turn over, neurons in the human cerebral neocortex are not generated in adulthood at detectable levels but are generated perinatally.
Keywords: neocortex, stem cell
It has remained controversial whether neurons are added to the cerebral neocortex in adult mammals. Some studies have suggested that neurogenesis persists in the adult rodent (1, 2) and monkey neocortex (3–5), whereas other studies have failed to detect neurogenesis (6–8) or have detected it only in response to an insult (9, 10).
There is a considerable degree of plasticity in the neocortex, enabling, for example, memory formation (11), and there is evidence of structural alterations resulting in detectable changes in volumes in distinct areas in the human cortex with age and in response to certain conditions (12, 13). Much of the plasticity can be accounted for by modulating preexisting cells and their connections, but it is important to determine whether neuronal turnover may contribute to neocortical plasticity in humans.
New neurons derived from endogenous stem or progenitor cells are continuously added to discrete regions of the adult mammalian brain. This may be important for processes requiring plasticity, such as memory formation (14), and new neurons have been suggested to replace lost cells after stroke and other insults (15, 16). Furthermore, neurogenesis has been implicated in the pathogenesis of human neurological and psychiatric diseases (17–20).
The most common way to detect neurogenesis is by the integration of labeled nucleotides, such as BrdU, but there are inherent risks of both false-positive and false-negative results, making room for controversy (21, 22). Moreover, there are difficulties in performing these types of studies in humans, and there is little BrdU-labeled material available for analysis. We have recently developed a new method to retrospectively determine the age of cells in humans by measuring 14C in DNA (23). The entry of cosmic rays into the atmosphere results in de novo generation of 14C, which is matched by radioactive decay (t1/2 = 5,730 years), resulting in stable steady-state atmospheric levels. A striking exception was caused by above-ground nuclear bomb tests during the Cold War, which produced an approximate doubling of 14C levels in the atmosphere from 1955 to 1963 that rapidly distributed around the globe (24, 25). After the 1963 Test Ban Treaty, there have been no significant above-ground high-yield nuclear detonations, and 14C levels have decreased nearly exponentially (26), not because of radioactive decay, but because of equilibration with the oceans and uptake in the biotope. 14C in the atmosphere reacts with oxygen to form CO2 and is taken up by plants in photosynthesis. Our consumption of plants and animals that live off plants results in 14C levels in the human body mirroring those in the atmosphere at any given time (23, 27–29). Because DNA is stable after a cell has gone through its last cell division, the 14C level in DNA serves as a date mark for when a cell was born and can be used to retrospectively birth date cells in humans (23).
Here we present a systematic analysis of cell turnover in the major areas of the human neocortex. We have retrospectively birth-dated neurons by measuring the level of 14C and have analyzed the brains of individuals that received BrdU. We failed to detect BrdU-labeled neurons and report that neocortical neurons have 14C levels corresponding to the atmospheric levels at the time of birth of the individual.
Results
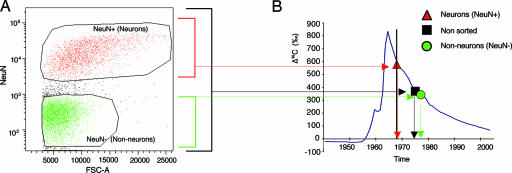
We have measured the 14C concentration in the DNA of cells in the major areas of the human cerebral neocortex by accelerator mass spectrometry (AMS). DNA was extracted from neurons and nonneuronal cells, respectively, after flow cytometric sorting of nuclei incubated with an antibody against the neuron-specific nuclear epitope NeuN (Fig. 1A). Flow cytometric gates were set to ensure the inclusion of all nuclei irrespective of size in the different populations, because adult-born cortical neurons in the rodent have been reported to be small (2). By comparing the measured 14C level in DNA to atmospheric concentrations at different times, we can establish the average year of birth for the cell populations (Fig. 1B; ref. 23).
Fig. 1.
Determination of the age of neocortical neurons. (A) Neuronal (NeuN-positive) and nonneuronal (NeuN-negative) cell nuclei from the adult human cerebral necortex were separated and isolated by flow cytometry. (B) The levels of 14C in the atmosphere have been stable over long time periods, with the exception of a large addition of 14C in 1955–1963 as a result of nuclear weapons tests (blue line, data from ref. 26), making it possible to infer the time of birth of cell populations by relating the level of 14C in DNA to that in the atmosphere (horizontal arrows) and reading the age off the x axis (vertical arrows). The average age of all cells in the prefrontal cortex is younger than the individual (black arrows), indicating cell turnover. Dating of nonneuronal cells demonstrates they are younger, whereas neurons are approximately as old as the individual. The vertical bar indicates the year of birth of the individual. 14C levels from modern samples are, by convention, given in relation to a universal standard and corrected for radioactive decay, giving the Δ14C value (50).
14C levels in DNA of neocortical cells from all lobes were analyzed, and the specific areas are indicated in Fig. 2A. Both prefrontal and premotor cortices were analyzed in the frontal lobe. We previously analyzed the occipital cortex, where a study had suggested neurogenesis in adult rats (1), but failed to detect any evidence for neurogenesis in this region in adult humans (23). In this study, we extended the analysis to the other lobes and, importantly, to regions where adult neurogenesis was reported in monkeys (4). We first studied individuals born after the Cold War and the Test Ban Treaty, because the decline in nuclear bomb test-derived 14C in the atmosphere during this period provides resolution as to when cells were born. Nonneuronal NeuN-negative cells always had 14C levels lower than those in the atmosphere at the time of birth of the individual, demonstrating cell turnover within this population (Fig. 2B and Supporting Text and Table 1, which are published as supporting information on the PNAS web site). These cells were on average born 4.9 ± 1.1 years (mean ± SEM, n = five measurements) after the birth of the individual. There are several possibilities as to how a population could have that average age, including, for example, that turnover is mainly restricted to childhood, or that the majority of nonneuronal cells are generated around birth and a subpopulation has a high turnover rate throughout life. It is not possible from this material to distinguish between these possibilities.
Fig. 2.
Neocortical neurons are as old as the individual. (A) The cerebral lobes are outlined (the large colored fields), and the cortical area analyzed within each lobe is color-coded. Both prefrontal (blue) and premotor (light blue) areas were analyzed in the frontal lobe. The analysis of occipital cortex was reported in ref. 23. (B) A representative example of values obtained from one individual born after the nuclear weapons tests plotted on to the curve of atmospheric 14C levels indicates that nonneuronal cells turn over, whereas the cortical neurons were generated close to the time of birth. (C) A representative example of the analysis of an individual born before the nuclear tests, indicating no measurable cortical neurogenesis. The 14C level in the nonneuronal cells demonstrates there is turnover within this population, but there are several possible interpretations of these data, and the age of this population cannot be concluded from this material alone. The coloring of symbols in B and C corresponds to the regions in A. Vertical bars in B and C indicate the birth date of the individual.
In contrast, the 14C levels in every analyzed sample of NeuN-positive neuronal nuclei from all individuals and all cortical regions showed 14C levels corresponding to close to the time of birth of the individual (Fig. 2B and Supporting Text). The cortical neurons were at 0.0 ± 0.4 years (mean ± SEM, n = five measurements), i.e., around the time of birth.
The strategy to birth date cells builds on the steep slope of 14C decline in the atmosphere after nuclear bomb tests. The resolution in time before the bomb tests is very poor. However, the low levels of 14C before the bomb pulse make the detection of a small population of cells born during or after the bomb tests especially sensitive, and a population constituting as little as 1% of the total cell population over the lifespan can be detected (23). 14C levels in DNA from NeuN-negative nonneuronal cells were invariably higher than prebomb levels in individuals born before the nuclear tests, again demonstrating turnover within this population (Fig. 2C and Supporting Text). However, because these 14C levels correspond to levels at the time of both increasing and decreasing 14C levels, the turnover rate of these populations cannot be inferred from these data alone. The 14C levels in DNA of NeuN-positive neurons, in contrast, corresponded to atmospheric levels before the nuclear bomb tests in all samples from all cortical regions in all individuals born before 1955 (Fig. 2C and Supporting Text). Thus, if there is any generation of stably integrating neocortical neurons during adulthood, they amount to <1% of the neuronal population up to the age of 72 years (the oldest individual included in the 14C analysis).
The 14C analysis provides cumulative information about the age of cells and about potential cell turnover over the lifespan of the individual. The analysis is therefore sensitive for the detection of very low-grade continuous generation of new cells that stably integrate and survive long term. However, if transitory cells are produced that are not maintained, they would remain unnoticed by this method if they constituted <1% of the cells at any given time. It has been suggested that neurons are generated in the adult monkey neocortex, but that they have a short lifespan and are transient (5), although other studies have not found evidence for the generation of short-lived neurons (6, 8). We next analyzed the neocortex of cancer patients that had received an injection of BrdU for diagnostic purposes (30). The time of death ranged between 4.2 months and 4.3 years after BrdU administration. As a positive control for the detection of adult-born neurons, we analyzed sections from the hippocampus from the same patients and were able to detect cells double-labeled with BrdU and NeuN in the granular layer of the dentate gyrus (30). In negative-control brains from individuals who did not receive a BrdU injection, we were unable to detect any BrdU labeling (data not shown).
BrdU-positive cells were disproportionately distributed through the depth of the motor cortex; 46% of the BrdU-positive cells were located in the white matter and <1–17% in the specific lamina (Fig. 3A). In total, in all patients studied, 515 BrdU-positive cells were identified in 205-mm3 tissue. We analyzed the identity of labeled cells in the frontal and motor cortexes by immunohistochemistry by using antibodies against cell type-specific markers. Less than 1% of the BrdU-positive cells were glia-like satellite cells, and a small subpopulation constituted GFAP-immunoreactive astrocytes (Fig. 3B). Most importantly, none of the BrdU-labeled cells had neuronal morphology or were immunoreactive to the neuronal markers NeuN or neurofilament. In cases where a BrdU-positive nucleus was located in close proximity to a NeuN- or neurofilament-immunoreactive neuron, 3D confocal reconstruction was performed to establish whether the labels coexisted in the same cell, but this was never the case (see examples in Fig. 3 C and D). Thus, we conclude that neurons are not generated in the adult human neocortex at levels detectable with the methods used, and if transient neurons are generated, they have a lifespan of <4.2 months.
Fig. 3.
BrdU incorporation in the adult human cerebral cortex. (A) Distribution of BrdU-labeled cells in the adult human motor cortex. (B) A subset of BrdU-labeled cells are immunoreactive to the astrocyte marker GFAP. (C and D) None of the BrdU-labeled cells are immunoreactive to the neuronal markers NeuN (C) or neurofilament (D). [Scale bars, 70 μm (B) and 100 μm (C and D).]
Discussion
Our analysis revealed that neurons in the adult human cerebral neocortex have 14C levels in their genomic DNA corresponding to atmospheric levels at the time when the individual was born, and we failed to detect BrdU-labeled neurons, which argues against postnatal cortical neurogenesis in humans.
It is important to underscore that both of our approaches to detect cell turnover in the adult neocortex have detection limits, and that we cannot exclude neurogenesis below this level. Retrospective 14C birth dating gives a cumulative measure that provides a high sensitivity to detect a low-grade continuous generation of new cells, even if these cells would account for only 1% of the neurons over the entire lifespan in the analyzed area (23). However, this requires that the newborn cells integrate stably and are maintained. It has been suggested that newborn neurons in the monkey neocortex have a short lifespan and are not maintained long term (5). If at any given time such neurons account for <1% of the neurons in the analyzed area, they would not be detectable by retrospective 14C birth dating with the current sensitivity.
In this context, BrdU labeling has the advantage that it labels newborn cells at a given point in time, and it would be easy to detect very much less than 1% of neurons being labeled at the time of analysis. The time period between BrdU administration and the death of the individuals we analyzed ranged between 4.2 months and 4.3 years. Our results thus indicate that there can be only little (<1%), if any, stable integration of cortical neurons in the adult human brain, and if there is a production of transitory neurons, they have a lifespan of <4.2 months.
We can, furthermore, integrate the information gained by retrospective birth dating and BrdU labeling to estimate the maximal level of adult neocortical neurogenesis that could remain unnoticed by the combination of both methods. With the established average age of all cells, the highest theoretical number of cells generated in adulthood would be if there are two populations, one generated around birth and the rest generated contemporarily. If we set the population generated before birth to be born at the time of birth (the average age of cortical neurons), we can calculate, based on the average age of all cells, that a population born during the last 5 years (on average 2.5 years before analysis) would constitute 37% of all cells in our population (as calculated for each individual given the known average age of all cells for that person; 37% represents the average of all individuals). Given our BrdU data, we know that maximally 1 of 516 adult-born cells could be neurons (in our study, 0 of 515 BrdU-labeled cells were seen to be neurons; however, we cannot rule out that if the sampling size had been larger, we may have detected BrdU+/NeuN+ cells, and thus we set the number of neurons as most being 1 of 516). Given that maximally 37% of the population was turning over in the last 5 years, we estimate that <0.07% (0.37 of 1 of 516) of the cells in the adult human neocortex could represent a neuron that was generated during the last 5 years and that was stably integrated.
Several studies have demonstrated the presence of cells with in vitro neural stem cell potential in the human cortex, including in subcortical white matter (31). Our results do not exclude the possibility that neocortical neurogenesis may occur in certain pathologies, or that it may be possible to induce it, as has been suggested in the rodent cortex (9, 10, 32). There is no or minimal neurogenesis in the rodent striatum under normal conditions, but large numbers of neurons are generated in response to growth-factor administration or stroke (15, 16, 33, 34). Although our current study indicates that neocortical neurogenesis does not take place in humans under normal conditions, it will be important to analyze whether there is a latent potential that results in neurogenesis in pathological situations.
There are clear species differences with regard to the extent of adult neurogenesis in vertebrates. Large numbers of neurons may be added throughout life in fish (35). However, fish often continue to grow, which could be viewed as a continuation of development. Substantial numbers of new neurons, including both interneurons and projection neurons, are added to several regions in birds such as zebra finches and canaries (36). In rodents, interneurons are added to the dentate gyrus of the hippocampus and to the olfactory bulb in mature animals (14). There are many reports indicating more low-grade neurogenesis in other areas of the rodent brain, but many of these studies await confirmation. The number of neurons that are added in the rodent hippocampus and olfactory bulb decreases substantially with age, although neurogenesis continues at low levels throughout life. 3H-thymidine studies originally indicated that there is less adult neurogenesis in the primate brain (37, 38), and later studies using BrdU have demonstrated relatively lower levels of neurogenesis in the dentate gyrus and olfactory bulb compared to rodents (39–41). One study demonstrated neurogenesis in the adult human dentate gyrus (30), but it remains controversial whether neurons are added to the adult human olfactory bulb (42, 43). Thus the distribution of adult neurogenesis appears to have been gradually more restricted with evolution, although there is still limited information available regarding the extent and distribution of neurogenesis in the adult human brain.
Plasticity is an important aspect of cortical function and is necessary, for example, for the integration of new memories. It is also easy to see the importance of stability for the maintenance of memories, for example. There must be a delicate balance between plasticity and stability, and the lack of human neocortical neurogenesis suggests that cellular stability has been favored.
Materials and Methods
Tissue Collection.
Tissues for 14C analysis were procured from cases admitted for autopsy during 2003 and 2004 to the Department of Forensic Medicine in Stockholm, Sweden, with the consent of relatives. Ethical permission for this study was granted by the Karolinska Institute Ethical Committee. Tissue from seven individuals born between 1933 and 1973 (five individuals born before and two born after the nuclear bomb tests) was analyzed for 14C content in this study. The cause of death was chest trauma (n = 1), hanging (n = 4), electrocution (n = 1), or myocardial infarction (n = 1). Tissues were frozen in 1-g samples and stored at −80°C until further analysis.
BrdU (250 mg in saline) was administered i.v. to assess the proliferative nature of tumor cells in patients diagnosed with squamous cell carcinoma at the base of the tongue, in the pharynx, or in the larynx. Metastatic spread of the carcinomas was not seen in the brain in any of these patients, and no anticancer therapy was administered before, during, or shortly after BrdU administration.
Flow Cytometry of Nuclei.
Nuclear isolation and flow cytometry were performed as described (23). NeuN antibodies (44) were directly conjugated with Zenon mouse IgG labeling reagent (Alexa 488; Molecular Probes Carlsbad, CA). To ensure that only single nuclei were sorted, an aliquot of nuclei was stained with DRAQ5, and the singlet population was plotted as a function of forward-scatter width vs. forward-scatter height. Using these parameters, it is easy to discriminate single nuclei from doublets, triplets, and potential higher-order aggregates as well as background noise (45). Nuclei were sorted based on purity, and purity of all sorts was confirmed by reanalyzing the sorted populations. Δ14C levels were corrected when purity was <100%. All FACS analysis and sorting were performed by using a FACSVantage DiVa (BD Biosciences, San Jose, CA). Nuclei pellets were collected by centrifugation and stored at −80°C until extraction with NaI, as described (23). DNA purity for all samples was analyzed by spectrophotometry and HPLC.
AMS.
All AMS analyses were performed blind to age and origin of the sample. Purified DNA samples suspended in water were transferred to quartz combustion tubes and evaporated to dryness in a convection oven maintained at 90–95°C. To convert the DNA sample into graphite, excess CuO and silver wire were added to each dry sample, and the tubes were evacuated and sealed with a H2/O2 torch. Tubes were placed in a furnace set at 900°C for 3.5 h to combust all carbon to CO2. The evolved CO2 was purified, trapped, and reduced to graphite in the presence of iron catalyst in individual reactors (46). Graphite targets were measured at the Center for Accelerator Mass Spectrometry at Lawrence Livermore National Laboratory and at the ANTARES AMS Facility at the Australian Nuclear Science and Technology Organisation (47, 48).
Large CO2 samples (>500 μg) were split, and δ13C was measured by stable isotope ratio mass spectrometry, which established the δ13C correction to –23 ± 2, which was applied for all samples. Corrections for background contamination introduced during sample preparation were made following the procedures of Brown and Southon (49). The measurement error was determined for each sample and ranged between ±2‰ and 10‰ (1 SD) Δ14C. All 14C data are reported as decay-corrected Δ14C following the dominant convention of Stuiver and Polach (50).
Detection of BrdU and Phenotypic Markers.
The brains from patients who received BrdU were removed, and the cortex and hippocampi were dissected, postfixed in 4% paraformaldehyde for 24 h, and then incubated in 30% sucrose until equilibrated. Sections were cut on a freezing-sledge microtome in the coronal plane and stored in cryoprotective buffer containing 25% ethylene glycol, 25% glycerin, and 0.05 M phosphate buffer. Free-floating sections were washed, incubated with HCl to denature DNA (51), and blocked 1 h in 3% human and 3% horse serum. The sections were incubated with antibodies against BrdU (1:200; Accurate, Westbury, NY), NeuN (1:50; Chemicon, Temecula, CA), neurofilament 200 (1:200; Sigma-Aldrich, Stockholm, Sweden), or GFAP (1:5,000; Dako, Copenhagen, Denmark) for 48 h, then washed and incubated for 12 h in goat anti-mouse Alexa 488 and goat anti-rat Alexa 594 (1:200; Molecular Probes). The sections were then washed and mounted onto glass slides and coverslipped by using Dako mounting medium. All together, 515 BrdU-labeled cells (in 205-mm3 tissue) were found.
Supplementary Material
Acknowledgments
We thank Q. Hua, P. Reimer, and K. Stenström for discussions on radiocarbon analysis; U. Zoppi and Mathew Josh for assistance in the AMS measurement; K. Hamrin and M. Toro for help with flow cytometry; K. Alkass for technical assistance; M. Stahlberg and T. Bergman for help with HPLC; and D. Kurdyla, P. Zermeno, and Alan Williams for producing graphite. This study was supported by grants from the Knut och Alice Wallenbergs Stiftelse, the Human Frontiers Science Program, the Swedish Research Council, the Juvenile Diabetes Research Foundation, the Swedish Cancer Society, the Foundation for Strategic Research, the Ingabritt och Arne Lundbergs Stiftelse, the Karolinska Institute, the Tobias Foundation, and the National Institutes of Health/National Center for Research Resources (Grant RR13461). This work was performed in part under the auspices of the U.S. Department of Energy by the University of California, Lawrence Livermore National Laboratory under contract W-7405-Eng-48 and the Australian Nuclear Science and Technology Organisation under contract AMS-05–02. R.D.B. was supported by a fellowship from the Parkinson Society Canada, and M.A.C. was supported by a Neurological Foundation of New Zealand, Wrightson Postdoctoral Fellowship.
Glossary
Abbreviation:
- AMS
accelerator mass spectrometry.
Footnotes
Conflict of interest statement: No conflicts declared.
See Commentary on page 12219.
References
- 1.Kaplan M. S. J. Comp. Neurol. 1981;195:323–338. doi: 10.1002/cne.901950211. [DOI] [PubMed] [Google Scholar]
- 2.Dayer A. G., Cleaver K. M., Abouantoun T., Cameron H. A. J. Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernier P. J., Bedard A., Vinet J., Levesque M., Parent A. Proc. Natl. Acad. Sci. USA. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gould E., Reeves A. J., Graziano M. S., Gross C. G. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 5.Gould E., Vail N., Wagers M., Gross C. G. Proc. Natl. Acad. Sci. USA. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornack D. R., Rakic P. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 7.Ehninger D., Kempermann G. Cereb. Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- 8.Koketsu D., Mikami A., Miyamoto Y., Hisatsune T. J. Neurosci. 2003;23:937–942. doi: 10.1523/JNEUROSCI.23-03-00937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magavi S. S., Leavitt B. R., Macklis J. D. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Magavi S. S., Macklis J. D. Proc. Natl. Acad. Sci. USA. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chklovskii D. B., Mel B. W., Svoboda K. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 12.Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U., May A. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 13.Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Evans A., Rapoport J., Giedd J. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 14.Falk A., Frisén J. Ann. Med. 2005;37:480–486. doi: 10.1080/07853890500371890. [DOI] [PubMed] [Google Scholar]
- 15.Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 16.Parent J. M., Vexler Z. S., Gong C., Derugin N., Ferriero D. M. Ann. Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 17.Sheline Y. I., Wang P. W., Gado M. H., Csernansky J. G., Vannier M. W. Proc. Natl. Acad. Sci. USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., et al. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 19.Duman R. S. Biol. Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson P. S. Exp. Neurol. 2006;199:26–27. doi: 10.1016/j.expneurol.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Nowakowski R. S., Hayes N. L. Science. 2000;288:771. doi: 10.1126/science.288.5467.771a. [DOI] [PubMed] [Google Scholar]
- 22.Rakic P. J. Neurosci. 2002;22:614–618. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spalding K., Bhardwaj R. D., Buchholz B., Druid H., Frisén J. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 24.De Vries H. Science. 1958;128:250–251. doi: 10.1126/science.128.3318.250. [DOI] [PubMed] [Google Scholar]
- 25.Nydal R., Lovseth K. Nature. 1965;206:1029–1031. doi: 10.1038/2061029a0. [DOI] [PubMed] [Google Scholar]
- 26.Levin I., Kromer B. Radiocarbon. 2004;46:1261–1272. [Google Scholar]
- 27.Harkness D. D. Nature. 1972;240:302–303. doi: 10.1038/240302a0. [DOI] [PubMed] [Google Scholar]
- 28.Libby W. F., Berger R., Mead J. F., Alexander G. V., Ross J. F. Science. 1964;146:1170–1172. doi: 10.1126/science.146.3648.1170. [DOI] [PubMed] [Google Scholar]
- 29.Spalding K. L., Buchholz B. A., Bergman L.-E., Druid H., Frisén J. Nature. 2005;437:333–334. doi: 10.1038/437333a. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson P. S., Perfilieva E., Björk-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., Gage F. H. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 31.Nunes M. C., Roy N. S., Keyoung H. M., Goodman R. R., McKhann G., 2nd, Jiang L., Kang J., Nedergaard M., Goldman S. A. Nat. Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 32.Nakatomi H., Kuriu T., Okabe S., Yamamoto S., Hatano O., Kawahara N., Tamura A., Kirino T., Nakafuku M. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 33.Benraiss A., Chmielnicki E., Lerner K., Roh D., Goldman S. A. J. Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pencea V., Bingaman K. D., Wiegand S. J., Luskin M. B. J. Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zupanc G. K. Brain Behav. Evol. 2001;58:250–275. doi: 10.1159/000057569. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez-Byulla A., Kirn J. R. Neurobiology. 1997;33:585–601. [PubMed] [Google Scholar]
- 37.Rakic P. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 38.Rakic P. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 39.Gould E., Reeves A. J., Fallah M., Tanapat P., Gross C. G., Fuchs E. Proc. Natl. Acad. Sci. USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kornack D. R., Rakic P. Proc. Natl. Acad. Sci. USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kornack D. R., Rakic P. Proc. Natl. Acad. Sci. USA. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanai N., Tramontin A. D., Quinones-Hinojosa A., Barbaro N. M., Gupta N., Kunwar S., Lawton M. T., McDermott M. W., Parsa A. T., Manuel-Garcia Verdugo J., et al. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 43.Bedard A., Parent A. Brain Res. Dev. Brain Res. 2004;151:159–168. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Mullen R. J., Buck C. R., Smith A. M. Development (Cambridge, U.K.) 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 45.Wersto R. P., Chrest F. J., Leary J. F., Morris C., Stetler-Stevenson M. A., Gabrielson E. Cytometry. 2001;46:296–306. doi: 10.1002/cyto.1171. [DOI] [PubMed] [Google Scholar]
- 46.Vogel J. S., Southon J. R., Nelson D. E. Nucl. Instrum. Methods Phys. Res. B. 1987;29:50–56. [Google Scholar]
- 47.Fink D., Hotchkis M., Hua Q., Jacobsen G., Smith A. M., Zoppi U., Child D., Mifsud C., van der Gaast H., Williams A., Williams M. Nucl. Instrum. Methods Phys. Res. B. 2004:223–224. 109–115. [Google Scholar]
- 48.Hua Q., Zoppi U., Williams A., Smith A. Nucl. Instrum. Methods Phys. Res. B. 2004:223–224. 284–292. [Google Scholar]
- 49.Brown T. A., Southon J. R. Nucl. Instrum. Methods Phys. Res. B. 1997;123:208–213. [Google Scholar]
- 50.Stuiver M., Polach H. A. Radiocarbon. 1977;19:355–363. [Google Scholar]
- 51.Kuhn P. G., Dickinson-Anson H., Gage F. H. J. Neurosci. 1996;16:20–27. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.