Abstract
Neuroimaging techniques are among the most important tools for investigating the function of the human nervous system and for improving the clinical diagnosis of neurological disorders. However, most commonly used techniques are limited by their invasiveness or their inability to accurately localize neural activity in space or time. Previous attempts at using MRI to directly image neuroelectric activity in vivo through the detection of magnetic field changes induced by neuronal currents have been challenging because of the extremely small signal changes and confounding factors such as hemodynamic modulations. Here we describe an MRI technique that uses oscillating magnetic field gradients to significantly amplify and detect the Lorentz effect induced by neuroelectric activity, and we demonstrate its effectiveness in imaging sensory nerve activation in vivo in the human median nerve during electrical stimulation of the wrist. This direct, real-time, and noninvasive neuroimaging technique may potentially find broad applications in neurosciences.
Keywords: Lorentz effect, median nerve, neuroimaging, MRI
The ongoing pursuit to better detect neural activity has led to many exciting technical advances over the past decades, including single-cell electrical recordings, electroencephalography (EEG), magnetoencephalography (MEG), positron emission tomography (PET), and, more recently, functional magnetic resonance imaging (fMRI). To date, neuroimaging techniques are invasive (single-cell recordings) and/or limited in their ability to accurately localize neural activity in space (EEG, MEG) or in time (PET, fMRI). Even when information is combined across multiple modalities, there remain fundamental limitations that introduce sources of error and interpretative difficulties. It is therefore time to develop novel techniques that allow noninvasive imaging of neural activity with a high accuracy both spatially and temporally.
Several studies have explored the feasibility of using MRI for detecting the minute magnetic field changes induced by electrical currents in phantoms (1–3) or by neuronal currents in human subjects (4–13), thereby combining the high temporal resolution of electrical and magnetic recording methods with the high spatial resolution and noninvasiveness inherent in MRI. Despite some encouraging results in phantoms, the direct imaging of neural activation in vivo has been challenging because of the small activation-induced magnetic field changes and because of multiple, synchronized, confounding signals in the brain reflecting cerebral blood oxygenation, blood volume, and blood flow changes or physiological noise (6, 9). Here we seek to address these two fundamental issues and to demonstrate the potential capability of MRI to directly image neuroelectric activity in vivo.
First, to boost the signal detectability, we propose an acquisition strategy based on the Lorentz effect induced by neuroelectric activity. The contrast mechanism of Lorentz effect imaging was initially validated in phantoms with a proof-of-concept pulse sequence (14); however, the original technique was not sensitive enough for in vivo applications. We therefore extended this technique by incorporating a series of oscillating magnetic field gradients applied in synchrony with the stimulation to drastically increase its sensitivity, and we recently demonstrated its feasibility for imaging electrical currents, on the order of microamperes, with a temporal resolution, on the order of milliseconds, in phantoms (15).
Second, to isolate neuroelectric activity from potential confounds, we performed our experiments in the human median nerve by using pulsed low-amplitude electrical stimulation of the wrist to induce intrinsic sensory nerve action potentials propagating proximally in the median nerve. This approach differs from that used in an early report (16) in which an external electrical current was injected into the muscles to cause a visible effect. Our well established sensory stimulation paradigm offers several advantages: a simple anatomical system that can be stimulated in isolation, precise timing characteristics derived from electrophysiological studies, and few confounds from hemodynamic modulations or physiological noise.
Our technique relies on the well known Lorentz effect, whereby a current-carrying conductor exposed to a magnetic field experiences a Lorentz force equal to the cross-product of the current vector and the magnetic field. If the conductor is surrounded by an elastic medium, this force induces a displacement of the conductor, resulting in a spatially incoherent displacement of the elastic medium in adjacent regions. In the presence of a magnetic field gradient, the spins in these regions experience a loss of phase coherence proportional to its amplitude and duration. This dephasing in turn results in a destructive signal summation within a voxel similar to the transverse relaxation effect, causing an exponential signal decay. As such, this contrast mechanism does not require the current to be unidirectional; in fact, a random current pattern will generate the same effect (15).
Here we apply a series of oscillating gradients (with positive and negative lobes of the same amplitude and duration) in synchrony with the neural stimulation, such that the neuroelectric activity occurs only during the negative lobes. Consequently, the loss of phase coherence of the spins experiencing the Lorentz effect is drastically amplified, whereas static spins remain unaffected, thus significantly enhancing the sensitivity of the technique (15).
Electrical stimulation of the median nerve was achieved by applying a series of 1 ms-long current pulses, triggered at the onset of the negative lobes of the oscillating gradients. The current amplitude was set below the motor threshold to ensure that the stimulation did not induce any muscle contraction, which was further confirmed by using electromyography measurements (see Methods). Because the conduction time of the sensory activation in the human median nerve between the wrist and the elbow is ≈4 ms (17), a duration of 5 ms was chosen for each lobe of the oscillating gradients to ensure that sensory nerve action potentials could propagate in that section of the median nerve within one lobe. A large number of oscillating gradients, and therefore a long echo time, should be used to generate sufficient accumulative loss of phase coherence and maximize the detectability of the Lorentz effect. However, a short echo time should be used to minimize the global signal attenuation due to T*;2 relaxation. Given these two competing constraints, we determined that three cycles of oscillating gradients, which can be accommodated within an echo time on the order of T*2 of human soft tissue, would be optimal.
The activation paradigm was a block design consisting of seven alternating rest and stimulation periods, each lasting 20 s, during which 10 image volumes were acquired. During the stimulation periods, electrical pulses were triggered to excite the median nerve, whereas during the rest periods, no stimulation was applied. Four runs were acquired for each study and averaged to increase the signal-to-noise ratio.
Results
Four different experiments were performed. In Exp. 1, three cycles of oscillating gradients and three electrical pulses synchronized with the negative gradient lobes were used for each image acquisition (Fig. 1A). Highly significant activation was found along the median nerve across subjects, as illustrated in the representative activation map shown in Fig. 2. The time course averaged over the activated region shows a systematic signal decrease of 4.4 ± 0.7% during the stimulation periods (Fig. 3A). The transitions between rest and stimulation periods exhibit no delay, in contrast to what is typically observed in conventional blood oxygenation level-dependent (BOLD) functional MRI (18) studies, which are limited by a hemodynamic delay of 3–6 s.
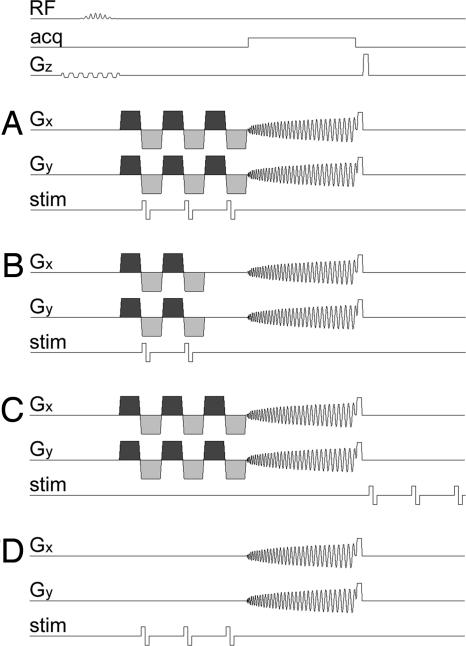
Fig. 1.
Pulse sequence diagrams. Timing of the radiofrequency excitation pulse (RF), the data acquisition window (acq), the magnetic field gradients on the slice selection (Gz), readout (Gx), and phase encoding (Gy) axes, and the current applied by the stimulator to the wrist (stim) for Exps. 1–4. (A) Three cycles of oscillating magnetic field gradients (shown in dark and light gray) and three electrical pulses triggered at the onset of the negative gradient lobes (Exp. 1). (B) The same as A but with two cycles of oscillating gradients and two electrical pulses (Exp. 2). (C) The same as A but with the electrical pulses delayed by 50 ms (Exp. 3). (D) The same as A but without oscillating gradients (Exp. 4).
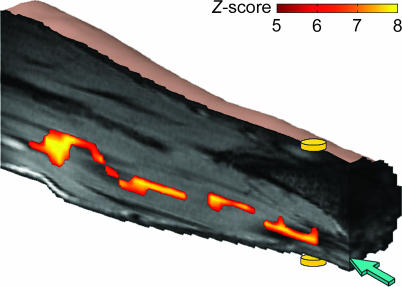
Fig. 2.
Activation map showing the effect of neuroelectric activity in vivo in the human median nerve. The activation was obtained by using three cycles of oscillating gradients and three electrical pulses synchronized with the negative gradient lobes (Exp. 1). The map is overlaid on a stack of coregistered anatomical images. The discs represent the electrodes placed on the dorsal (upper) and ventral (lower) sides of the wrist. The arrow points to the median nerve.
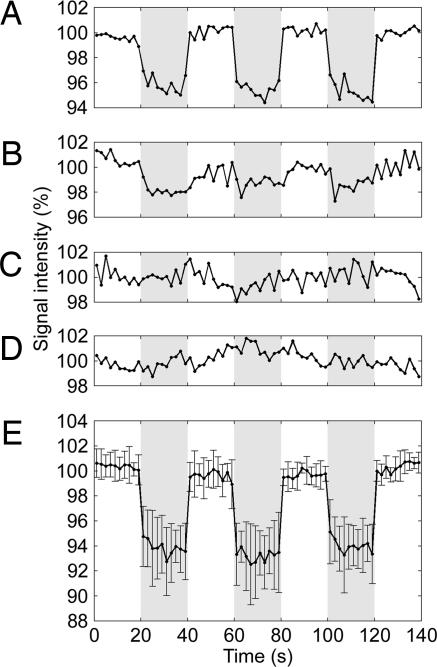
Fig. 3.
Time courses during alternating periods of rest and electrical stimulation. These results were obtained by using the following methods. (A) Three cycles of oscillating gradients and three electrical pulses synchronized with the negative gradient lobes (Exp. 1). (B) The same as A but with two cycles of oscillating gradients and two electrical pulses (Exp. 2). (C) The same as A but with the electrical pulses delayed by 50 ms (Exp. 3). (D) The same as A but without oscillating gradients (Exp. 4). (E) The same as A but averaged over seven different experimental sessions. The time courses in A–D were averaged over the activated region obtained in Exp. 1; the time course in E was averaged over the activated regions of all seven sessions. The error bars represent the standard deviation over the seven sessions. Each time course was normalized to the mean signal intensity during rest. The rest and stimulation periods are shown in white and gray, respectively.
Exp. 2 was identical to Exp. 1, except that only two cycles of oscillating gradients and two electrical pulses synchronized with the negative gradient lobes were used (Fig. 1B). Less significant activation was detected. The time course averaged over the same activated region as in Exp. 1 shows a signal decrease of 1.5 ± 0.6% during the stimulation periods (Fig. 3B). This finding illustrates that the sensitivity of our technique can be significantly decreased when there is insufficient loss of phase coherence, consistent with its signal loss mechanism.
Exps. 3 and 4 were control experiments identical to Exp. 1 but with the electrical pulses delayed by 50 ms with respect to the oscillating gradients (Fig. 1C) or without oscillating gradients (Fig. 1D), respectively. No activation was detected in either experiment. The time courses averaged over the same activated region as in Exp. 1 show no systematic signal changes during the stimulation periods (Fig. 3 C and D). These results further validate the contrast mechanism of our technique by demonstrating that the observed activation is indeed due to the loss of phase coherence generated by the oscillating gradients rather than to the displacement of the nerve alone, because this displacement is present in both control experiments but is not synchronized with oscillating gradients. It should thus be emphasized that the magnitude of the displacement does not directly determine the sensitivity of the technique, because, for a given displacement, the loss of phase coherence can be independently amplified by using more, stronger, and/or longer oscillating gradients, given a sufficient signal-to-noise ratio. Furthermore, the fact that the loss of phase coherence can be amplified by the oscillating gradients demonstrates that it is indeed caused by the spatially incoherent displacement rather than by the magnetic field induced by the current. Finally, Exps. 3 and 4 also show that there are no artifacts due to eddy currents induced in the stimulation circuit by the oscillating gradients or due to electrical interference from the stimulation pulses, respectively.
It can therefore be derived that Exp. 2 is equivalent to Exp. 1 with a one-cycle (i.e., 10-ms) temporal offset between the electrical stimulation and the oscillating gradients, because only electrical pulses that are synchronized with oscillating gradients contribute to the observed activation. As such, the significant difference between the results of Exps. 1 and 2 shows that our technique is sensitive to temporal offsets of the stimulation paradigm on the order of milliseconds, thus demonstrating its high temporal resolution.
Finally, to evaluate the test–retest reliability of our technique for directly imaging neuroelectric activity in vivo in a healthy median nerve, Exp. 1 was carried out in seven separate experimental sessions on the same subject under identical experimental conditions to remove the dependence on subject and experimental variability. (To establish this subject as a representative healthy subject, we first performed independent event-related potential measurements at different locations along the median nerve by using the same electrical stimulation paradigm as in the MRI experiments, and we obtained a mean conduction velocity consistent with the literature.) Highly significant activation was consistently detected along the median nerve in each session. The time course in the activated regions averaged over the seven sessions (Fig. 3E) highly resembles that obtained in a single session (Fig. 3A) and shows a systematic signal decrease of 6.3 ± 2.2% during the stimulation periods, thus demonstrating the consistency and robustness of our technique.
Discussion
In this work, we have demonstrated the capability of an MRI acquisition strategy for imaging neuroelectric activity in vivo in the human median nerve. Such a direct, real-time, and noninvasive neuroimaging technique may find broad applications in neurosciences. It has the potential to characterize nerve conductivity in various neuropathies, unlike traditional imaging methods that only reveal anatomical differences. Furthermore, it can potentially be applied to white matter tracts in the central nervous system to study the functional connectivity between various brain areas and to assess white matter integrity in diseases such as multiple sclerosis. Last, because our technique does not require the electrical current to be unidirectional, it can potentially be extended to image dendritic neuroelectric activity in gray matter, which, if successful, would have a large impact on the study of neuronal information processing in the brain. However, unlike for applications in the peripheral nervous system, the temporal delays between the stimuli and the neural activation in various cortical areas are often not known, making it difficult to synchronize the pulse sequence with the neuroelectric activity. Nevertheless, scalp event-related potential recordings can be used to determine the proper delays, which can then be incorporated into the pulse sequence to allow time-locked detection of neural activation.
The successful detection of neuroelectric activity in vivo by using our technique demonstrates that neural activation can be imaged noninvasively by MRI with a high spatial and temporal resolution (on the order of milliseconds). This finding should stimulate further development of this emerging field to have a greater impact on neuroscience research.
Methods
Stimulus.
Electrical stimulation of the median nerve was achieved by using a high-impedance electrical current stimulator (Grass S12; Grass-Telefactor, West Warwick, RI) and two gold-plated disk electrodes secured on the ventral and dorsal sides of the right wrist directly over the median nerve. (Identical results were obtained when both electrodes were placed on the ventral side of the wrist directly over the median nerve.) The current was delivered through the filtered penetration panel through shielded and twisted cables, with the shield grounded to the panel. All electrical switches were installed outside the magnet room, effectively isolating the electrical stimulation in the magnet room and thus removing any electrical interference with the MRI signal. The stimuli consisted of a series of biphasic rectangular current pulses with a duration of 1 ms and an amplitude ranging from 1.8 to 2.7 mA, which was set before each session to be just below the motor threshold for finger movement to avoid motion artifacts. The stimulator was triggered by the MRI scanner to ensure an accurate synchronization between the electrical stimulation and the pulse sequence.
To verify that our median nerve sensory stimulation paradigm did not induce any muscle contraction that could potentially confound the studies, we performed electromyography measurements from the flexor carpi radialis and flexor carpi ulnaris during three different conditions: rest, electrical stimulation of the median nerve, and maximal voluntary isometric contraction. The results obtained during rest and electrical stimulation were identical, whereas those obtained during isometric muscle contraction showed a maximal signal two orders of magnitude larger, thus confirming the absence of muscle contraction induced by the electrical stimulation (Fig. 4, which is published as supporting information on the PNAS web site).
Data Acquisition.
The studies were performed on a 4 T whole-body MRI scanner (General Electric Medical Systems, Milwaukee, WI) to increase the sensitivity of the technique, because the magnitude of the Lorentz effect and the signal-to-noise ratio both increase with field strength. In repeated sessions, two healthy volunteers, who gave written informed consent as approved by our Institutional Review Board, were instructed to lie prone in the scanner with the right forearm placed superior to the head and orthogonal to the main magnetic field. After high-order shimming, images were acquired by using an in-house-designed surface coil, a gradient echo single-shot spiral imaging sequence (with the following parameters: repetition time, 2,000 ms; echo time, 35.5 ms; flip angle, 80°; field-of-view, 20 cm; matrix size, 64 × 64), and three contiguous axial slices (sagittal with respect to the forearm), which were 15-mm thick and centered on the median nerve to ensure that the section of the median nerve between the wrist and the elbow would be fully contained within one slice. A series of oscillating gradients with a maximum achievable amplitude of 36 mT/m and a duration of 5 ms for each lobe was applied along both axes orthogonal to the main magnetic field. High-resolution T1-weighted images were also acquired at the same location for anatomical reference.
Data Analysis.
For each run, subject bulk motion was assessed by computing the displacement of the image center-of-mass over time slice by slice, and runs with an in-plane displacement exceeding one pixel were discarded (≈10% of the runs). Voxel-by-voxel linear detrending was applied to remove any linear drift in the MRI signal. A group Student’s t test (one-tailed) was then carried out to detect significant differences between the images acquired during the rest and stimulation periods. The t score maps were converted to Z score maps and thresholded by using a Z score of Z > 5 (corresponding to a significance level of P < 2.5 × 10−7 uncorrected for multiple comparisons) and a cluster size of five voxels. Finally, the resulting activation maps were overlaid on the coregistered, high-resolution anatomical images.
Supplementary Material
Acknowledgments
We thank G. McCarthy for his help in implementing the median nerve stimulation paradigm; N. Goutkin and J. Tozer for their assistance with the MRI scanning; R. Segal and C. Lin for performing the electromyography measurements; A. Perkins and T. Grent-’t-Jong for performing the event-related potential measurements; and S. Huettel, T. Harshbarger, C. Weingarten, and M. Woldorff for helpful discussions and comments on the manuscript. This work was supported in part by National Science Foundation Grant BES 602529 and National Institutes of Health Grant NS 50329.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Bodurka J., Jesmanowicz A., Hyde J. S., Xu H., Estkowski L., Li S. J. J. Magn. Reson. 1999;137:265–271. doi: 10.1006/jmre.1998.1680. [DOI] [PubMed] [Google Scholar]
- 2.Bodurka J., Bandettini P. A. Magn. Reson. Med. 2002;47:1052–1058. doi: 10.1002/mrm.10159. [DOI] [PubMed] [Google Scholar]
- 3.Konn D., Gowland P., Bowtell R. Magn. Reson. Med. 2003;50:40–49. doi: 10.1002/mrm.10494. [DOI] [PubMed] [Google Scholar]
- 4.Kamei H., Iramina K., Yoshikawa K., Ueno S. IEEE Trans. Magn. 1999;35:4109–4111. [Google Scholar]
- 5.Xiong J., Fox P. T., Gao J. H. Hum. Brain Mapp. 2003;20:41–49. doi: 10.1002/hbm.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu R., de Zwart J. A., van Gelderen P., Fukunaga M., Kellman P., Holroyd T., Duyn J. H. NeuroImage. 2004;23:1059–1067. doi: 10.1016/j.neuroimage.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Bianciardi M., Di Russo F., Aprile T., Maraviglia B., Hagberg G. E. Magn. Reson. Imaging. 2004;22:1429–1440. doi: 10.1016/j.mri.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Konn D., Leach S., Gowland P., Bowtell R. Magn. Reson. Imaging. 2004;22:1413–1427. doi: 10.1016/j.mri.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Bandettini P. A., Petridou N., Bodurka J. Appl. Magn. Reson. 2005;29:65–88. [Google Scholar]
- 10.Chow L. S., Cook G. G., Whitby E., Paley M. N. J. NeuroImage. 2006;30:835–846. doi: 10.1016/j.neuroimage.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Hagberg G. E., Bianciardi M., Maraviglia B. Magn. Reson. Imaging. 2006;24:483–493. doi: 10.1016/j.mri.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Xue Y., Gao J.-H., Xiong J. NeuroImage. 2006;31:550–559. doi: 10.1016/j.neuroimage.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Chow L. S., Cook G. G., Whitby E., Paley M. N. J. Magn. Reson. Imaging. 2006;24:681–691. doi: 10.1016/j.mri.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Song A. W., Takahashi A. M. Magn. Reson. Imaging. 2001;19:763–767. doi: 10.1016/s0730-725x(01)00406-4. [DOI] [PubMed] [Google Scholar]
- 15.Truong T.-K., Wilbur J. L., Song A. W. J. Magn. Reson. 2006;179:85–91. doi: 10.1016/j.jmr.2005.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joy M., Scott G., Henkelman M. Magn. Reson. Imaging. 1989;7:89–94. doi: 10.1016/0730-725x(89)90328-7. [DOI] [PubMed] [Google Scholar]
- 17.Kimura J. Electrodiagnosis in Disease of Nerve and Muscle: Principles and Practice. Oxford: Oxford Univ. Press; 2001. [Google Scholar]
- 18.Ogawa S., Menon R. S., Tank D. W., Kim S. G., Merkle H., Ellermann J. M., Ugurbil K. Biophys. J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.