Abstract
Photosystem II (PSII) catalyzes the first of two photosynthetic reactions that convert sunlight into chemical energy. Native PSII is a supercomplex consisting of core and light-harvesting chlorophyll proteins. Although the structure of PSII has been resolved by x-ray crystallography, the mechanism underlying its assembly is poorly understood. Here, we report that an immunophilin of the chloroplast thylakoid lumen is required for accumulation of the PSII supercomplex in Arabidopsis thaliana. The immunophilin, FKBP20-2, belongs to the FK-506 binding protein (FKBP) subfamily that functions as peptidyl-prolyl isomerases (PPIases) in protein folding. FKBP20-2 has a unique pair of cysteines at the C terminus and was found to be reduced by thioredoxin (Trx) (itself reduced by NADPH by means of NADP-Trx reductase). The FKBP20-2 protein, which contains only two of the five amino acids required for catalysis, showed a low level of PPIase activity that was unaffected on reduction by Trx. Genetic disruption of the FKBP20-2 gene resulted in reduced plant growth, consistent with the observed lower rate of PSII activity determined by fluorescence (using leaves) and oxygen evolution (using isolated chloroplasts). Analysis of isolated thylakoid membranes with blue native gels and immunoblots showed that accumulation of the PSII supercomplex was compromised in mutant plants, whereas the levels of monomer and dimer building blocks were elevated compared with WT. The results provide evidence that FKBP20-2 participates specifically in the accumulation of the PSII supercomplex in the chloroplast thylakoid lumen by means of a mechanism that has yet to be determined.
Keywords: chloroplast thylakoid lumen, protein folding, photosynthetic electron transport
Much of life on Earth is sustained by oxygenic photosynthesis, a process that utilizes sunlight to produce oxygen and organic carbon from water and carbon dioxide. The absorption of light and its conversion into chemical energy is brought about by two photosystems [photosystem II (PSII) and photosystem I (PSI)] acting sequentially. PSII catalyzes the light-dependent oxidation of water that results in the evolution of oxygen. The electrons released in this reaction are transferred along a photosynthetic electron transport chain that leads, by means of PSI, to the production of NADPH and ATP, the chemical energy currency used for carbon fixation.
The chloroplast PSII core complex consists of ≈17 protein subunits that include the D1 and D2 reaction centers for chlorophyll (Chl) P680 binding, cytochrome b559, CP43 and CP47 for building the Chl antennae, and other proteins whose function is less well characterized (1–4). The native form of PSII residing in the thylakoid membrane is believed to be a supercomplex consisting of the core and peripheral light-harvesting complex II (LHCII) components. The light harvested by LHCII is transferred to the core complex that brings about charge separation, thereby driving the transfer of electrons from water to plastoquinone and initiating photosynthetic electron transport. Recent studies using x-ray and cryoelectron crystallography have resolved the three-dimensional structure of the PSII complexes in both cyanobacteria and land plants (5–9), providing a platform for further elucidation of PSII’s properties.
Despite our advanced understanding of its structure and function, relatively little is known about molecular mechanisms underlying the biogenesis and maintenance of the PSII supercomplex. It is established that biogenesis of the complex involves the translation of chloroplast as well as nuclear-encoded protein subunits, the latter imported from the cytosol. Although much work has investigated the pathways and mechanisms of import into both the stroma and the lumen (10, 11), aside from a study that identified a thylakoid-associated protein involved in PSII biogenesis (12), little is known about how the protein subunits are assembled. It is certain that interaction among different subunits is required during assembly, because mutation of a single subunit gene resulted in mutants lacking PSII (13–16). Further, a number of proteins that participate in assembly and maintenance, such as molecular chaperones, most likely act specifically on one side of the thylakoid membrane (e.g., at either the stromal or luminal face).
Earlier studies on PSII assembly concentrated on the role of stromal factors, such as the translation and import machinery, because only a limited number of proteins were considered to reside in the thylakoid lumen. However, the number of possible luminal participants in assembly has increased dramatically because of recent proteomic findings that suggest a population of 80–100 proteins in that compartment (17–19). One of the predominant groups identified is the immunophilin family made up of FKBP (FK-506 binding protein) and cyclophilin members. Originally defined as receptors for immunosuppressive drugs (FK506 and cyclosporin A) (20), these proteins are now known to occur widely and function as protein foldases and chaperones. The identification of at least 16 immunophilins in the thylakoid lumen suggests that these protein-folding catalysts play a critical role in the assembly and maintenance of protein complexes such as the two photosystems that reside at least in part in the thylakoid lumen (21–23).
To pursue this possibility, we have applied a systematic genetic approach to dissect the function of lumen immunophilins in the context of photosynthesis. We now report that an FKBP-type immunophilin localized in the thylakoid lumen, FKBP20-2, functions in the accumulation of the PSII supercomplex in Arabidopsis.
Results and Discussion
Isolation of fkbp20-2 Mutants of Arabidopsis.
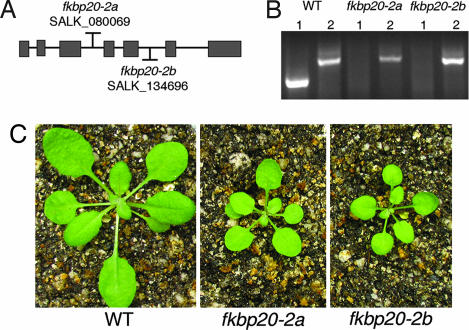
All immunophilins of the thylakoid lumen are transcribed from the nuclear genome, translated in the cytosol, and targeted to the lumen as shown in several studies (17, 18, 24). To characterize the function of this family of proteins, we isolated multiple transfer DNA (T-DNA) insertional mutants for each of the relevant immunophilin genes. We then screened the mutants for potential growth defects under short-day conditions (10-h light/14-h dark cycle with light intensity of 180 μmol of photons·m−2·s−1). Two independent mutant alleles for the gene encoding a 20-kDa FKBP, FKBP20-2, showed stunted growth. The T-DNA insertional alleles (SALK_080069 and SALK_134696), referred to as fkbp20-2a and fkbp20-2b, contained T-DNA insertions located in introns 3 and 5 (Fig. 1A). The mRNA of FKBP20-2 was undetectable by RT-PCR in both mutants (Fig. 1B), indicating that the T-DNA insertions (fkbp20-2a and 2b) had disrupted the expression of the FKBP20-2 gene. Under normal growth conditions, leaves of both mutants were much smaller and less green than the WT (Fig. 1C). Chl analysis revealed a reduction in Chl of ≈25% per leaf area for both mutants. (Chl a: fkbp20-2, 16.6 ± 1.4 μg/cm2; WT, 22.2 ± 1.7 μg/cm2. Chl b: fkbp20-2, 5.4 ± 1.1 μg/cm2; WT, 7.5 ± 0.4 μg/cm2.) By contrast, the Chl a/b ratio was the same in mutant and WT. The fact that the two independent mutants showed a similar phenotype suggested that the defect in plant growth was caused by disruption of FKBP20-2 gene expression. The experiments described below were carried out with both of the fkbp20-2 mutants. Although data are presented for only one mutant (fkbp20-2b) for simplicity, the results were consistently the same with the other mutant (fkbp20-2a) within experimental error.
Fig. 1.
Genetic characterization and phenotype of FKBP20-2 mutant plants. (A) Localization of the T-DNA insertions in the two mutant alleles. (B) RT-PCR amplification of FKBP20-2 mRNA (lane 1) and ACTIN2 control (lane 2). The WT and mutant alleles are indicated above the gel. (C) Phenotype of the two fkbp20-2 mutants and WT plants 6 weeks after planting.
Amino Acid Sequence Analysis and Enzymatic Activity of FKBP20-2.
The FKBP20-2 protein has a single FKBP domain with a clear thylakoid lumen targeting signal (designated in Fig. 2 by a box showing the arginine residues and a line marking the hydrophobic component), consistent with proteomic analyses localizing FKBP20-2 to that compartment (17, 18). Sequence analysis revealed homologs in cyanobacteria as well as algae, suggesting conservation among oxygenic photosynthetic organisms (Fig. 2). Further, the positioning of two conserved Cys residues located at the C terminus of the algal and land plant sequences suggests the presence of a disulfide bridge that would be absent in the cyanobacterial homologs (see arrows in Fig. 2).
Fig. 2.
Comparison of FKBP20-2 and related protein sequences. The amino acid sequence of A. thaliana (At3g60307) FKBP20-2 was aligned with homologs in rice (O. sativa, Q7XHRO), a green alga (C. reinhardtii, gene model C_2101108), and a cyanobacterium (S. elongatus, Q8DJW8). Sequences were aligned with ClustalW (38). The line indicates the hydrophobic stretch after the two arginine residues (boxed) that constitute the derived thylakoid targeting sequence (21). Arrows indicate conserved cysteine residues in land plants and the green alga that are absent in the cyanobacterial proteins. Asterisks indicate positions essential for PPIase activity (25).
Protein mutational analysis with the human FKBP12 protein revealed five amino acids that are required for PPIase activity (identified with an asterisk for the homologs shown in Fig. 2) (25). All five are conserved in FKBP13, whereas FKBP20-2 possesses only two of the essential amino acids (positions D155 and F217 shown for the Arabidopsis protein in Fig. 2).
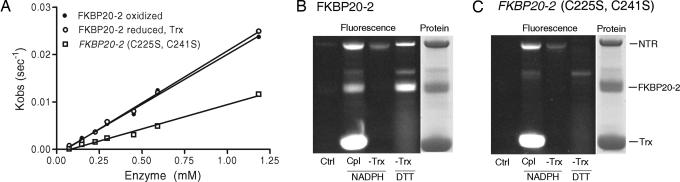
To determine whether FKBP20-2 could act as a foldase and whether the conserved cysteines influence function, we assayed the enzyme for PPIase activity by using a modification of the protocol (26) adopted from the original procedure of Kofron et al. (27). As seen in Fig. 3A, WT FKPB20-2 was enzymatically active (kcat/Km = 0.021 μM−1·s−1). However, its activity was ≈1/500 of that observed with FKBP13 (28), a possible reflection of the above-noted deficiency in residues required for activity. A similar deficiency can explain the apparent lack of activity of another Arabidopsis immunophilin, FKBP42 (29). In this case, four of the five required amino acids described above were missing from the active site of the enzyme.
Fig. 3.
Enzymatic and redox activity of FKBP20-2. (A) PPIase activity. The values here have been corrected for controls lacking the FKBP20-2 enzyme. The rates shown represent first-order rate fits. (B and C) Reduction of FKBP20-2 by the NADP/Trx system from E. coli. Ctrl, control (FKBP20-2 alone); Cpl, complete Trx system (NADPH plus NTR plus Trx plus FKBP20-2); -Trx, complete Trx system omitting Trx; DTT, DTT alone. “Protein” refers to a Coomassie blue stain of the complete Trx system. (B) FKBP20-2. (C) FKBP20-2 (C225S, C241S).
Mutations in the cysteine residues reduced the PPIase activity by 50% (kcat/Km = 0.01 μM−1·s−1), suggesting that these residues may be involved in catalysis or regulation (Fig. 3A). FKBP20-2 also resembled FKBP13 in being reduced by Escherichia coli thioredoxin (Trx), itself reduced by NADPH and NADP-Trx reductase (NTR) (Fig. 3B). Negligible reduction was observed with reduced glutathione (data not shown). As expected, the FKBP20-2 (C225S, C241S) mutant showed no response to reduced Trx or DTT (Fig. 3C). Although the redox properties of FKBP20-2 and FKBP13 were similar, the enzymes differed with respect to the effect of reduction. The activity of FKBP20-2 was unaffected by Trx reduced either by NADPH and NTR (Fig. 3A) or DTT (not shown), whereas that of FKBP13 was decreased (28). The low PPIase activity coupled with the disconnect between Cys to Ser mutagenesis (50% inhibition of activity) and disulfide reduction (no effect on activity) raises the question of the true enzymatic function of FKBP20-2. Although the question remains open, it is noted that disulfide reduction and Cys-to-Ser mutagenesis were reported to give contrasting effects with a stromal cyclophilin (30). Another unanswered question concerns the significance of reduction of FKPB20-2 by Trx. As discussed for another immunophilin, FKBP13, reduction by Trx, taking place in the stroma, could facilitate transport into the lumen (28, 31). Once in the lumen, the protein would be oxidized to the disulfide form. It is possible that these proteins are also reduced in the lumen by a disulfide protein that is present in that compartment.
Analysis of fkbp20-2 Mutants.
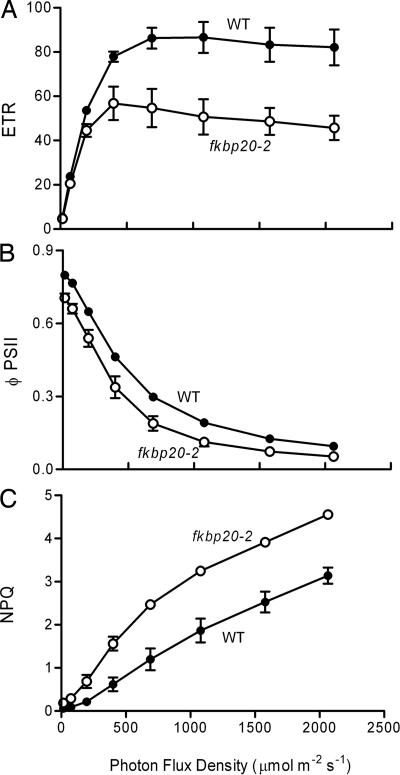
The observation that the FKBP20-2 protein is located in the thylakoid lumen and that mutants lacking the protein were stunted suggested that the mutants might have defects in photosynthesis. Photosynthetic parameters were initially determined by measuring Chl fluorescence in the leaves of WT and mutant plants. Electron transport rates (ETRs) were found to be similar in the two plants for light intensities up to 200 μmol of photons·m−2·s−1 (Fig. 4A). However, when the light intensity reached 500 μmol of photons·m−2·s−1 or higher, the ETR of the mutant was significantly (approximately one-third) lower than WT, suggesting a defect in the electron transport chain. At higher intensities, the difference became more pronounced. That the problem might reside at the level of PSII was suggested by the finding that the efficiency of PSII electron transport (φPSII) estimated as a function of incident photon flux density was also lower in mutant plants (Fig. 4B). By contrast, nonphotochemical quenching was higher in mutant plants than in WT plants (Fig. 4C), likely a result of the need for increased dissipation of excess energy because of lower PSII activity and photochemical quenching. The maximum efficiency of PSII photochemistry (Fv/Fm) was also decreased in mutant plants (data not shown). Taken together, these results suggested that fkbp20-2 mutants had defects specifically related to PSII function.
Fig. 4.
Light response curves for Chl fluorescence parameters. Data are means ± SE, n = 3. (A) Electron transport rates (ETR). (B) Efficiency of PSII electron transport (φPSII). (C) Nonphotochemical quenching (NPQ).
PSII was further analyzed by measuring ferricyanide-dependent oxygen evolution with isolated chloroplast thylakoid membranes. As observed in the fluorescence experiments, activity in the fkbp20-2 mutant was significantly reduced (approximately one-third) relative to WT (Fig. 5). This finding is significant in that it confirms the fluorescence data (based on mutant leaves containing less Chl per leaf area than WT) with an independent oxygen evolution assays (based on equal Chl concentrations). The results with both approaches were consistent with a defect in PSII.
Fig. 5.
Ferricyanide-dependent O2 evolution from WT and fkbp20-2 mutant plants. Chloroplasts isolated from 12-week-old plants were used in these experiments. (A) Rates of O2 evolution. Data are means ± SE, n = 9. (B) Dependence of O2 evolution on light. ↑, light on; ↓, light off.
Function of FKBP20-2.
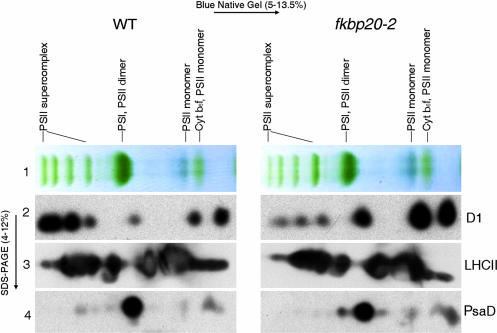
Although they suggested a defect in PSII function, the above results gave no indication as to the nature of the problem. We, therefore, examined the composition of PSII in mutants and WT plants by using a native gel procedure to separate and quantify the photosynthetic protein complexes. Isolated thylakoid membranes were treated with dodecyl maltoside to solubilize the photosynthetic supercomplexes that were then loaded at equal protein and Chl concentration onto blue native gels and separated under nondenaturing conditions (Fig. 6). The results revealed specific differences between the mutant and WT in the abundance of the PSII supercomplex. The higher-molecular-weight green bands corresponding to components of the supercomplex appeared less intense in the mutant (Fig. 6, row 1). Bands for the PSII monomer and dimer, on the other hand, showed increased accumulation in the mutant relative to WT. Immunoblot scans revealed the following ratios of D1 for mutant relative to WT: PS supercomplex, 38%; PSI, PSII dimer, 585%; PSII monomer, 326%. These differences provide additional evidence that the mutant has a defect in PSII.
Fig. 6.
Composition of chloroplast thylakoid protein complexes determined by blue native gel and immunoblot analysis. Row 1 shows resolution of the WT and fkbp20-2 mutant complexes under native (nondenaturing) conditions. Rows 2–4 show immunoblots of WT (Left) and fkbp20-2 (Right) proteins resolved under denaturing conditions. Antibodies were applied as indicated.
To confirm and quantify these differences, we applied immunoblot analysis to examine key components of the two photosystems and the cytochrome b6 f complex. The individual blue native gel lanes were subjected to SDS/PAGE in a second dimension to separate individual protein components in the complexes. Using antibodies against D1, the PSII reaction center protein, immunoblots clearly showed a reduced accumulation of this protein associated with the PSII supercomplex in fkbp20-2 mutants (Fig. 6, row 2). By contrast, the levels of the D1 protein in the lower-molecular-weight components (the monomers and dimers not incorporated in the PSII supercomplex) were higher in the mutant. The distribution of LHCII (light-harvesting complex II) proteins was not altered (Fig. 6, row 3). Thus, the results indicated a change in supercomplex stoichiometry in the mutant: less D1 and the same amount of LHCII. The PsaD protein of the PSI complex (Fig. 6, row 4) and the cytochrome f of the Cytb6 f complex (data not shown) showed no significant differences in mutant and WT.
Concluding Remarks.
As the catalyst of the first of two photoreactions in the chloroplast electron transport chain, PSII converts water into oxygen and a source of electrons (for the ultimate reduction of ferredoxin and NADP) and protons (for the synthesis of ATP). The composition of this impressive protein complex has been largely elucidated. Many of the components are well characterized, and recent structural analysis has revealed the three-dimensional topology of the complex. A key question that remains is how this complex, as well as its companion catalyzing the PSI photoreaction, is assembled and functionally maintained. Both complexes contain proteins that are synthesized in the cytosol and chloroplast stroma and are ultimately transported to the thylakoid membrane or luminal space, where they are assembled. The present study shows that FKBP20-2, an FKBP-type immunophilin of the thylakoid lumen, is essential for the accumulation of the PSII supercomplex. Understanding the mechanism underlying this function will be facilitated by identifying the immediate target(s) of FKBP20-2.
FKBP20-2 contains a disulfide bridge at the C terminus and was reduced by Trx. Interestingly, this disulfide bridge is highly conserved in land plants and eukaryotic algae but is absent in cyanobacteria, indicating that potential redox regulation is restricted to chloroplasts. However, the low level of PPIase activity associated with FKBP20-2 did not appear to be affected after reduction by Trx, raising the question of whether PPIase activity is the key property responsible for the accumulation (assembly or maintenance) of the PSII complex. This question assumes special interest in view of the recent finding that the PPIase activity of FKBP13, another immunophilin of the thylakoid lumen, is regulated by a redox system (28, 31).
Materials and Methods
Plant Materials.
All genotypes used were of the Arabidopsis ecotype Columbia-0. AtFKBP20-2 (At3g60307) insertional mutants (SALK_080069 and SALK_134696) were isolated from the T-DNA-transformed Arabidopsis collection at the Arabidopsis Biological Resource Center (Columbus, OH). (For simplicity, we refer to the AtFKBP20-2 mutants as fkbp20-2a and fkbp20-2b.) Primers used for genomic PCR verification of T-DNA insertion in the mutants were 5′-GGG AGG ATC CAT GGT GAC GAT TCT ATC AAC TCC-3′ and 5′-CAT TCT CGA GTT AAC TGC ATG TGA CAT CTG AGT-3′. The left border primer used was 5′-GCG TGG ACC GCT TGC TGC AAC TCT CT-3′. Confirmation of null mutants was carried out by RT-PCR using the same primer set described above for FKBP20-2. Expression levels of ACTIN2 were monitored as a quantifying control (5′-GGA AGG ATC TGT ACG GTA AC-3′ and 5′-TGT GAA CGA TTC CTG GAC CT-3′). For use in the experiments described below, plants were grown for 12 weeks in a 10-h light (22°C)/14-h dark (20°C) cycle with a photon flux density of 180 μmol of photons·m−2·s−1.
Amino Acid Sequence Alignment.
The amino acid sequence of FKBP20-2 was aligned with homologs in rice (Oryza sativa, Q7XHRO), a green alga (Chlamydomonas reinhardtii, gene model C_2101108), and a cyanobacterium (Synechococcus elongatus, Q8DJW8). The targeting peptide was determined by the methods of He et al. (21).
Isolation of Thylakoid Membranes.
Thylakoid membranes were isolated as described in ref. 32 with minor modifications. For blue native gel analyses, leaves were homogenized in ice-cold homogenization buffer (330 mM sorbitol/30 mM Mops-KOH, pH 7.8/2 mM EDTA/2 mM ascorbate) and filtered through two layers of Miracloth (Calbiochem, San Diego, CA). The filtrate was centrifuged (at 2,000 × g for 2 min at 4°C). The pellet was resuspended in homogenization buffer, overlaid on 40% Percoll solution [40% (wt/vol) Percoll/330 mM sorbitol/30 mM Mops-KOH, pH 7.8/2 mM EDTA/2 mM ascorbate) and centrifuged (at 2,500 × g for 15 min at 4°C). Isolated thylakoids were resuspended in the same buffer to a final Chl concentration of 0.4 mg/ml (33).
Blue Native Gel Electrophoresis.
Blue native gel electrophoresis (34) was performed as described in ref. 35 with modification. Isolated thylakoids (0.4 mg/ml Chl) were solubilized with an equal volume of 50 mM Bis-Tris·HCl, pH 7.0/1.5 M ε-aminocaproic acid/15% (wt/vol) glycerol containing 1.5% (wt/vol) N-dodecyl-β-d-maltoside (Sigma-Aldrich, St. Louis, MO) and incubated for 10 min on ice. Samples were centrifuged (at 10,000 × g for 10 min at 4°C). After centrifugation, loading buffer [5% (wt/vol) Serva Blue G (Serva, Heidelberg, Germany)/100 mM Bis-Tris·HCl, pH 7.0/0.5 M ε-aminocaproic acid/75% (wt/vol) glycerol] was added to the supernatant solution (1/10 volume). For both mutant and WT, 18 μl of sample (corresponding to 88 μg of protein and 7.0 μg of Chl) was loaded to a blue native gradient gel containing 5–13.5% polyacrylamide (30:0.8 acrylamide:Bis) (Bio-Rad, Hercules, CA). Electrophoresis was performed in a MiniPROTEAN 3 cell (Bio-Rad) at 200 V at 4°C. The cathode buffer initially contained 0.01% Serva Blue G dye and was replaced by buffer lacking dye halfway through the run.
Two-Dimensional Immunoblot Analysis.
After electrophoresis, lanes of WT and mutant samples on blue native gel were excised with a razor blade and incubated for 10 min at 25°C in NuPAGE sample buffer (Invitrogen, Carlsbad, CA) supplemented with 2.5% 2-mercaptoethanol. Each lane was then placed on top of a 1-mm NuPAGE Bis-Tris 4–12% gel (Invitrogen) and subjected to second-dimension separation at constant voltage (110 V) at 25°C for 60 min. After separation in the second dimension, proteins were transferred to a nitrocellulose membrane (Bio-Rad). Membranes were preincubated in PBS containing 0.1% Tween 20 and 5% skimmed powdered milk. Blots were incubated overnight with primary antibody against D1, PsaD, and Cytf at 4°C. Chicken (D1) and rabbit (PsaD and Cytf) immunoglobulins coupled to horseradish peroxidase were used as secondary antibodies. Immunoblots were visualized by using the ECL Plus Western Blotting Detection System (Amersham Pharmacia Biosciences, Buckinghamshire, U.K.). The blots were scanned with a desktop scanner and analyzed by using Bio-Rad Quantity One.
Chl Fluorescence Assays.
Chl fluorescence measurements were performed with dark-adapted (overnight) plants by using an FMS2 instrument (Hansatech, King’s Lynn, U.K.). Six samples (three independently grown sets of WT and fkbp20-2 plants) were measured. Plants were subjected to a saturating light pulse and then illuminated for a programmed series of 5-min periods of increasing light intensities. Between steps in actinic photon flux density, the minimum fluorescence in the light-adapted state (Fo′) was determined during a 1-s period of far-red illumination. Nonphotochemical quenching is defined as (Fm − Fm′)/Fm′, efficiency of PSII electron transport (φPSII) is defined as (Fm′ − Fs)/Fm′, and the maximum efficiency of PSII photochemistry (Fv/Fm) is (Fm − Fo)/F, where Fm is the maximum fluorescence in the dark-adapted state, Fm′ is the maximum fluorescence in any light-adapted state, and Fs is the steady-state value of fluorescence immediately before the light flash.
Oxygen Evolution.
Thylakoid membranes were isolated as above with the following modifications. All procedures were carried out at 4°C under dim light. Leaves were homogenized in ice-cold homogenization buffer (10 mM NaCl/5 mM MgCl2/330 mM sorbitol/30 mM Mops-KOH, pH 7.8/2 mM EDTA). Chloroplasts were used directly after filtration and centrifugation. Thylakoids equivalent to 20 μg/ml Chl were resuspended in homogenization buffer. Potassium ferricyanide (500 μM) was added as an electron acceptor. O2 evolution was carried out at 25°C in a DW1 Clark Electrode (Hansatech) in response to increasing photon flux densities. Nine samples were measured (three independently grown sets of plants with three samples each).
Cloning, Expression, and Purification of Recombinant FKBP20-2.
The cDNA region encoding the FKBP20-2 mature protein was amplified from total mRNA isolated from leaf tissue of WT Arabidopsis thaliana. Primers used were 5′-GGG AGG ATC CAT GGT GAC GAT TCT ATC AAC TCC-3′ and 5′-CAT TCT CGA GTT AAC TGC ATG TGA CAT CTG AGT-3′. The mutant form of the protein lacking both cysteines at the C-terminal region was constructed by site-directed mutagenesis with the following primer sets: 5′-TCA GAT GTC ACA TCC AGT TAA CTC GAG-3′ and 5′-CTC GAG TTA ACT GGA TGT GAC ATC TGA-3′; 5′-CTC AGT ATC CAG AAT TCT GAG AGG AGG ACT ATA-3′ and 5′TAT AGT CCT CCT AGA ATT CTG GAT ACT GAG-3′. Amplification products were cloned into the expression vector pGEX-4T (Amersham Pharmacia Biosciences). Ultracompetent Rosetta E. coli cells were transformed according to the manufacturer’s instructions (Novagen, Madison, WI). Cultures were grown for 18 h at 25°C in ZYP-5052 (36). Cells were collected by centrifugation (at 10,000 × g for 15 min at 4°C), resuspended in PBS solution, and lysed by sonication (Fisher 300, Dynatech Labs, Chantilly, VA). Recombinant proteins were purified by affinity chromatography by using glutathione Sepharose beads (Amersham Pharmacia Biosciences). Fusion proteins were cleaved by thrombin and further purified by using 5,000 and 50,000 molecular-weight cutoff Vivaspin columns (Amersham Pharmacia Biosciences). Purified proteins were analyzed by 4–12% SDS/PAGE.
Enzyme Assay.
PPIase was assayed (27) at 20°C as in ref. 26. Activity was followed by the increase on absorbance at 390 nm due to release of p-nitroanilide by the proteolytic cleavage of the chromogenic substrate N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (N- succinyl-AAPF-p-nitroanilide) (Sigma-Aldrich). The reaction mixture contained 30 mM Tris (pH 7.0), 71 μM N- succinyl-AAPF-p-nitroanilide, 0.68 μM α-chymotrypsin (Sigma-Aldrich), and 0.15% 2,2,2 trifluorethanol. N-Succinyl-AAPF-p-nitroanilide was dissolved in 2,2,2 trifluorethanol containing 250 mM LiCl. Progress curves were fit to first-order rate constants (Kobs, s−1), and the second-order rate constant (kcat/Km) was determined from the slope of a plot of FKBP20-2 concentration vs. Kobs. FKBP20-2 and FKBP20-2 were reduced by Trx, which was itself reduced by the NADP/Trx system of E. coli (added in the same ratios as described below) or by DTT (added at 2.5 mM).
Redox Analysis of FKBP20-2 Protein.
Reduction of FKBP20-2 by the NADP/Trx system of E. coli was performed as described by Wong et al. (37). Recombinant FKBP20-2 and FKBP20-2 (C225S, C241S) (4.5 μg) proteins were incubated in reaction buffer (50 mM Tris·HCl, pH 7.5) at 37°C for 60 min. The complete sample contained 2.5 mM NADPH, 5 μg NTR, and 5 μg Trx. Newly exposed cysteines resulting from disulfide reduction were labeled by addition of the thiol-specific fluorescent probe monobromobimane to 2 mM. After labeling, the protein sample was incubated with 10 mM 2-mercaptoethanol for 2 min and was then subjected to electrophoresis on NuPAGE Bis-Tris (Invitrogen) using 4–12% gels. Fluorescence was recorded by using a Gel Doc-1000 fitted with a UV 365-nm Transilluminator and the Quantity One data analysis program (Bio-Rad). Subsequently, proteins in the gels were visualized by staining with colloidal Coomassie blue G-250.
Acknowledgments
We thank K. Niyogi, L. Curatti, Y. Balmer, Y. H. Cheong, A. Melis, and J. R. Dominguez for helpful discussions and R. Malkin (University of California, Berkeley, CA) for providing antibodies. This work was supported by grants from the U.S. Department of Agriculture National Research Initiative (to B.B.B. and S. Luan) and the U.S. Department of Energy (to S. Luan).
Glossary
Abbreviations
- Chl
chlorophyll
- Trx
thioredoxin
- NTR
NADP-Trx reductase
- PPIase
peptidyl-prolyl isomerase
- PSI
photosystem I
- PSII
photosystem II
- T-DNA
transfer DNA.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Erickson J. M. In: The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Rochaix J. D., Goldschmidt-Clermont M., Merchant S., editors. Dordrecht, Germany: Kluwer Academic; 1998. pp. 255–285. [Google Scholar]
- 2.Vermaas W. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993;44:457–481. [Google Scholar]
- 3.Baena-Gonzalez E., Aro E. M. Philos. Trans. R. Soc. London. 2002;357:1451–1460. doi: 10.1098/rstb.2002.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson N., Yocum C. F. Annu. Rev. Plant Biol. 2006;57:521–565. doi: 10.1146/annurev.arplant.57.032905.105350. [DOI] [PubMed] [Google Scholar]
- 5.Boekema E. J., Hankamer B., Bald D., Kruip J., Nield J., Boonstra A. F., Barber J., Rogner M. Proc. Natl. Acad. Sci. USA. 1995;92:175–179. doi: 10.1073/pnas.92.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zouni A., Witt H.-T., Kern J., Fromme P., Krauss N., Saenger W., Orth P. Nature. 2001;309:739–743. doi: 10.1038/35055589. [DOI] [PubMed] [Google Scholar]
- 7.Biesiadka J., Loll B., Kern J., Irrgang K.-D., Zouni A. Phys. Chem. Chem. Phys. 2004;6:4733–4736. [Google Scholar]
- 8.Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J., Iwata S. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 9.Kern J., Loll B., Zouni A., Saenger W., Irrgang K.-D., Biesiadka J. Photosynth. Res. 2005;84:153–159. doi: 10.1007/s11120-004-7077-x. [DOI] [PubMed] [Google Scholar]
- 10.Schnell D. J., Hebert D. N. Cell. 2003;4:491–505. doi: 10.1016/s0092-8674(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 11.Jarvis P., Robinson C. Curr. Biol. 2004;14:1064–1067. doi: 10.1016/j.cub.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Penga L., Maa J., Chia W., Guoa J., Zhua S., Lua Q., Lua C., Zhang L. Plant Cell. 2006;18:955–969. doi: 10.1105/tpc.105.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen K. H., Herrin D. L., Plumley F. G., Schidt G. W. J. Cell Biol. 1986;103:1315–1325. doi: 10.1083/jcb.103.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson J. M., Rahire M., Malnoë P., Girard-Bascou J., Pierre Y., Bennoun P., Rochaix J. D. EMBO J. 1986;5:1745–1754. doi: 10.1002/j.1460-2075.1986.tb04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vitry C., Olive J., Drapier D., Recouvreur M., Wollman F. A. Cell Biol. 1989;109:991–1006. doi: 10.1083/jcb.109.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pakrasi H. B., Nyhus K. J., Granok H. Z. Naturforsch. 1990;45:423–429. doi: 10.1515/znc-1990-0519. [DOI] [PubMed] [Google Scholar]
- 17.Peltier J. B., Emanuelsson O., Kalume D. E., Ytterberg J., Friso G., Rudella A., Liberles D. A., Soderberg L., Roepstroff P., von Heijne G. Plant Cell. 2002;14:11–266. doi: 10.1105/tpc.010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schubert M., Petersson U. A., Haas B. J., Funk C., Schroder W. P., Kieselbach T. J. Biol. Chem. 2002;277:8354–8365. doi: 10.1074/jbc.M108575200. [DOI] [PubMed] [Google Scholar]
- 19.Spetea C., Hundal T., Lundin B., Heddad M., Adamska I., Andersson B. Proc. Natl. Acad. Sci. USA. 2004;101:1409–1414. doi: 10.1073/pnas.0308164100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber S. L. Science. 1991;251:238–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 21.He Z. Y., Li L., Luan S. Plant Physiol. 2004;134:1248–1267. doi: 10.1104/pp.103.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano P., He Z., Luan S. Plant Physiol. 2004;134:1241–1243. doi: 10.1104/pp.103.900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romano P., Horton P., Gray J. E. Plant Physiol. 2004;134:1268–1282. doi: 10.1104/pp.103.022160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta R., Mould R., He Z., Luan S. Proc. Natl. Acad. Sci. USA. 2002;99:15806–15811. doi: 10.1073/pnas.222550399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Cenzo M., Park T. S., Jarrett B. P., Aldape R. A., Futer O., Murko M. A., Livingston D. J. Protein Eng. 1996;9:173–180. doi: 10.1093/protein/9.2.173. [DOI] [PubMed] [Google Scholar]
- 26.Jordens J., Janssens V., Longin S., Stevens I., Martens E., Bultynck G., Engelborghs Y., Lescrinier E., Waelkens E., Goris J., van Hoof C. J. Biol. Chem. 2006;281:6349–6357. doi: 10.1074/jbc.M507760200. [DOI] [PubMed] [Google Scholar]
- 27.Kofron J. L., Kuzmic P., Kishore V., Colon-Bonilla E., Rich D. H. Biochemistry. 1991;30:6127–6134. doi: 10.1021/bi00239a007. [DOI] [PubMed] [Google Scholar]
- 28.Gopalan G., He Z. Y., Balmer Y., Romano P., Gupta R., Herous A., Buchanan B. B., Swaminathan K., Luan S. Proc. Natl. Acad. Sci. USA. 2004;101:13945–13950. doi: 10.1073/pnas.0405240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamphausen T., Fanghanel J., Neumann D., Schulz B., Rahfeld J. U. Plant J. 2002;32:263–276. doi: 10.1046/j.1365-313x.2002.01420.x. [DOI] [PubMed] [Google Scholar]
- 30.Motahashi K., Koyama F., Nakanishi Y., Veoka-Nakanishi H., Hisabori T. J. Biol. Chem. 2003;278:31848–31852. doi: 10.1074/jbc.M304258200. [DOI] [PubMed] [Google Scholar]
- 31.Buchanan B. B., Luan S. J. Exp. Bot. 2005;56:1439–1447. doi: 10.1093/jxb/eri158. [DOI] [PubMed] [Google Scholar]
- 32.Bartlett S. G., Grossman A. R., Chua N.-H. In: Methods in Chloroplast Molecular Biology. Edelman M., Hallick R. B., Chua N.-H., editors. Amsterdam: Elsevier Biomedical; 1982. pp. 1081–1092. [Google Scholar]
- 33.Arnon D. I. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schägger H., Cramer W. A., von Jagow G. Anal. Biochem. 1994;217:220–230. doi: 10.1006/abio.1994.1112. [DOI] [PubMed] [Google Scholar]
- 35.Asakura Y., Hirohashi T., Kikuchi S., Belcher S., Osborne E., Yano S., Terashima I., Barkanb A., Nakai M. Plant Cell. 2003;16:201–214. doi: 10.1105/tpc.014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Studier F. W. Protein Expr. Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Wong J. H., Balmer Y., Cai N., Tanaka C. K., Vensel W. H., Hurkman W. J., Buchanan B. B. FEBS Lett. 2003;547:151–156. doi: 10.1016/s0014-5793(03)00696-3. [DOI] [PubMed] [Google Scholar]
- 38.Thompson J. D., Higgins D. G., Gibson T. J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]