Abstract
DNA damage induced by various reactive oxygen species can be characterized using a set of repair endonucleases with defined substrate specificities. DNA damage profiles thus obtained in a cell-free system can be compared with those observed in cellular DNA. Using this approach, we have demonstrated that an illumination of Salmonella typhimurium cells with visible light in the presence of methylene blue gives rise to a DNA damage profile very similar to that of singlet oxygen in a cell-free system. Therefore, the genotoxicity observed under these conditions most probably is attributable to the direct action of this species. The damage consists mainly of base modifications that are subject to repair by uvrABC-independent pathways. Revertant frequencies observed in parallel in the strains TA100 and TA2638 indicate a pronounced mutagenicity of the lesions induced. Exposure of Salmonella typhimurium to tert-butylhydroperoxide gives rise to another form of damage profile that is also different from that produced by hydroxyl radicals in a cell-free system. However, the latter dissimilarity does not exclude hydroxyl radicals as ultimate reactive species, as a very rapid repair of the induced base modifications is observed, which might have distorted the damage profile despite immediate work up.
Full text
PDF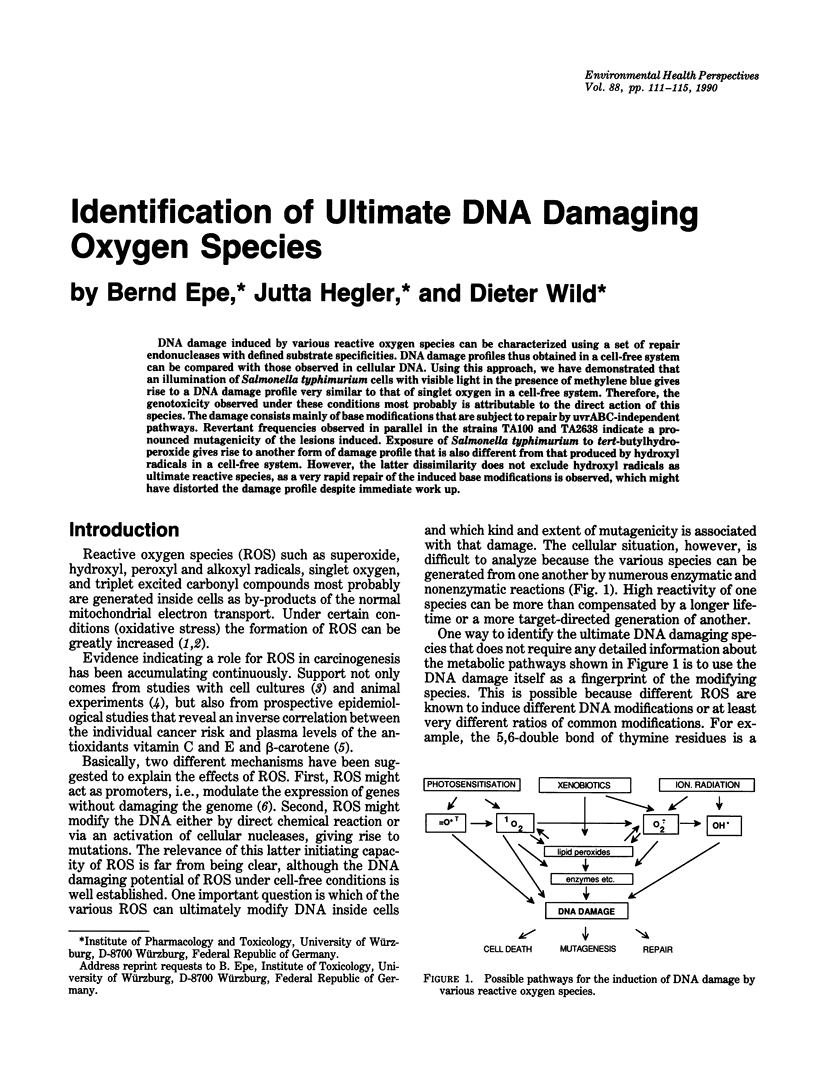

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Dahl T. A., Midden W. R., Hartman P. E. Pure exogenous singlet oxygen: nonmutagenicity in bacteria. Mutat Res. 1988 Sep;201(1):127–136. doi: 10.1016/0027-5107(88)90119-4. [DOI] [PubMed] [Google Scholar]
- Dahl T. A., Midden W. R., Hartman P. E. Pure singlet oxygen cytotoxicity for bacteria. Photochem Photobiol. 1987 Sep;46(3):345–352. doi: 10.1111/j.1751-1097.1987.tb04779.x. [DOI] [PubMed] [Google Scholar]
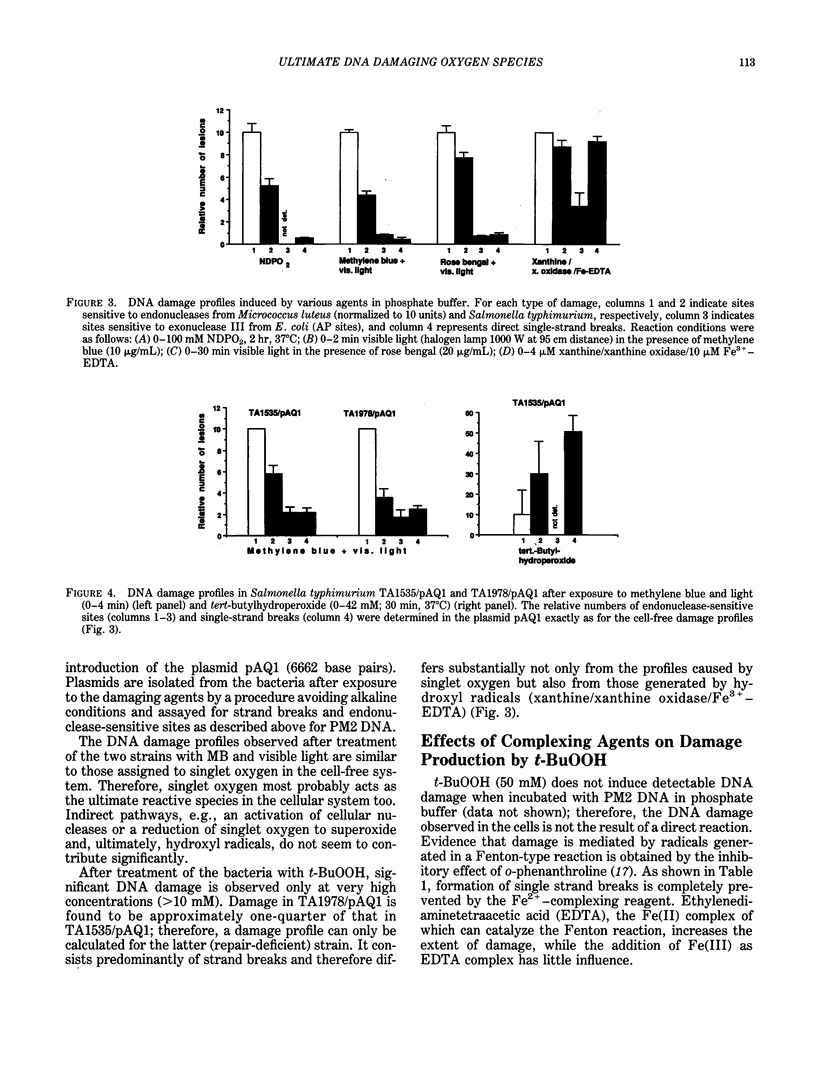
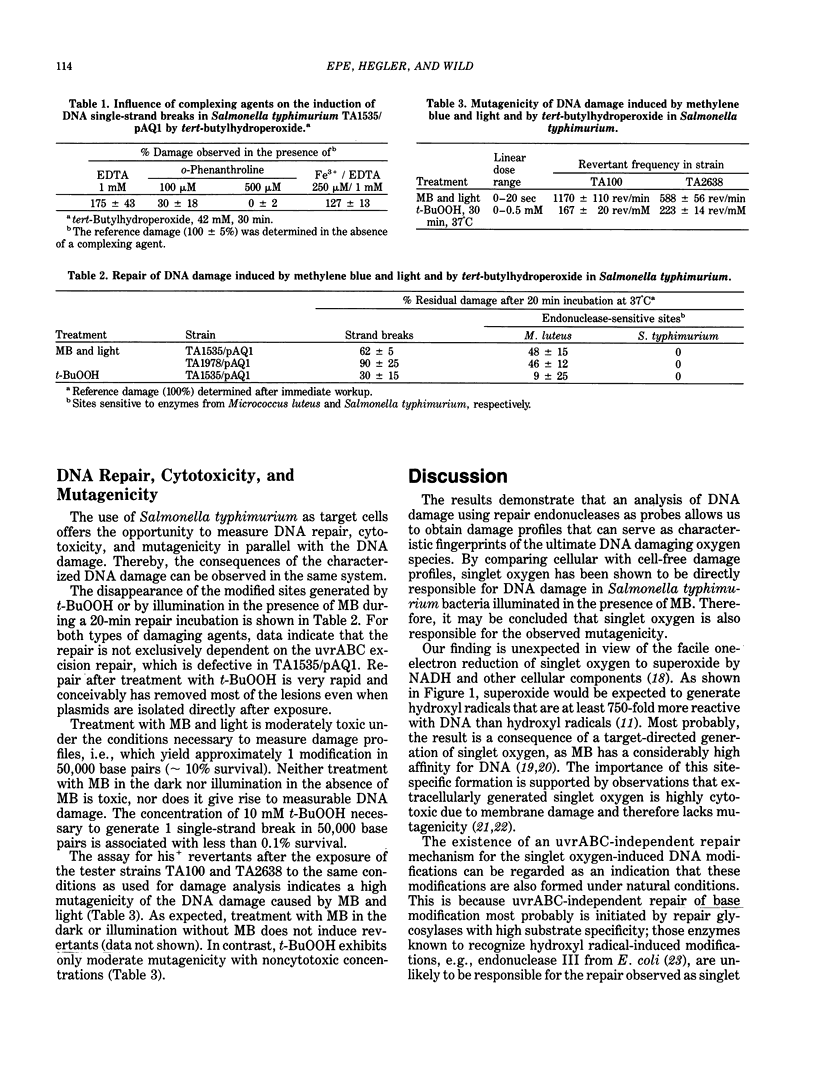
- Epe B., Hegler J., Wild D. Singlet oxygen as an ultimately reactive species in Salmonella typhimurium DNA damage induced by methylene blue/visible light. Carcinogenesis. 1989 Nov;10(11):2019–2024. doi: 10.1093/carcin/10.11.2019. [DOI] [PubMed] [Google Scholar]
- Epe B., Mützel P., Adam W. DNA damage by oxygen radicals and excited state species: a comparative study using enzymatic probes in vitro. Chem Biol Interact. 1988;67(1-2):149–165. doi: 10.1016/0009-2797(88)90094-4. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Brown D. M. Base-specific reactions useful for DNA sequencing: methylene blue--sensitized photooxidation of guanine and osmium tetraoxide modification of thymine. Nucleic Acids Res. 1978 Feb;5(2):615–622. doi: 10.1093/nar/5.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Katcher H. L., Wallace S. S. Characterization of the Escherichia coli X-ray endonuclease, endonuclease III. Biochemistry. 1983 Aug 16;22(17):4071–4081. doi: 10.1021/bi00286a013. [DOI] [PubMed] [Google Scholar]
- Linn S., Imlay J. A. Toxicity, mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Cell Sci Suppl. 1987;6:289–301. doi: 10.1242/jcs.1984.supplement_6.19. [DOI] [PubMed] [Google Scholar]
- Lloyd R. S., Haidle C. W., Robberson D. L. Bleomycin-specific fragmentation of double-stranded DNA. Biochemistry. 1978 May 16;17(10):1890–1896. doi: 10.1021/bi00603a014. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Hoffmann M. E., Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochem J. 1984 Feb 15;218(1):273–275. doi: 10.1042/bj2180273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlematter D., Larsson R., Cerutti P. Active oxygen induced DNA strand breakage and poly ADP-ribosylation in promotable and non-promotable JB6 mouse epidermal cells. Carcinogenesis. 1988 Feb;9(2):239–245. doi: 10.1093/carcin/9.2.239. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Colburn N. H., Gindhart T. D. Role of reactive oxygen in tumor promotion: implication of superoxide anion in promotion of neoplastic transformation in JB-6 cells by TPA. Carcinogenesis. 1985 Feb;6(2):229–235. doi: 10.1093/carcin/6.2.229. [DOI] [PubMed] [Google Scholar]
- Nieuwint A. W., Aubry J. M., Arwert F., Kortbeek H., Herzberg S., Joenje H. Inability of chemically generated singlet oxygen to break the DNA backbone. Free Radic Res Commun. 1985;1(1):1–9. doi: 10.3109/10715768509056532. [DOI] [PubMed] [Google Scholar]
- Ochi T., Cerutti P. A. Clastogenic action of hydroperoxy-5,8,11,13-icosatetraenoic acids on the mouse embryo fibroblasts C3H/10T1/2. Proc Natl Acad Sci U S A. 1987 Feb;84(4):990–994. doi: 10.1073/pnas.84.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OhUigin C., McConnell D. J., Kelly J. M., van der Putten W. J. Methylene blue photosensitised strand cleavage of DNA: effects of dye binding and oxygen. Nucleic Acids Res. 1987 Sep 25;15(18):7411–7427. doi: 10.1093/nar/15.18.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. G., Weiss B. Exonuclease III of Escherichia coli K-12, an AP endonuclease. Methods Enzymol. 1980;65(1):201–211. doi: 10.1016/s0076-6879(80)65028-9. [DOI] [PubMed] [Google Scholar]
- Stähelin H. B., Gey K. F., Brubacher G. Plasma vitamin C and cancer death: the prospective Basel Study. Ann N Y Acad Sci. 1987;498:124–131. doi: 10.1111/j.1749-6632.1987.tb23756.x. [DOI] [PubMed] [Google Scholar]
- Taffe B. G., Takahashi N., Kensler T. W., Mason R. P. Generation of free radicals from organic hydroperoxide tumor promoters in isolated mouse keratinocytes. Formation of alkyl and alkoxyl radicals from tert-butyl hydroperoxide and cumene hydroperoxide. J Biol Chem. 1987 Sep 5;262(25):12143–12149. [PubMed] [Google Scholar]
- Troll W., Wiesner R. The role of oxygen radicals as a possible mechanism of tumor promotion. Annu Rev Pharmacol Toxicol. 1985;25:509–528. doi: 10.1146/annurev.pa.25.040185.002453. [DOI] [PubMed] [Google Scholar]
- Wang J., Hogan M., Austin R. H. DNA motions in the nucleosome core particle. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5896–5900. doi: 10.1073/pnas.79.19.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werts E. D., Gould M. N. Relationships between cellular superoxide dismutase and susceptibility to chemically induced cancer in the rat mammary gland. Carcinogenesis. 1986 Jul;7(7):1197–1201. doi: 10.1093/carcin/7.7.1197. [DOI] [PubMed] [Google Scholar]


