Abstract
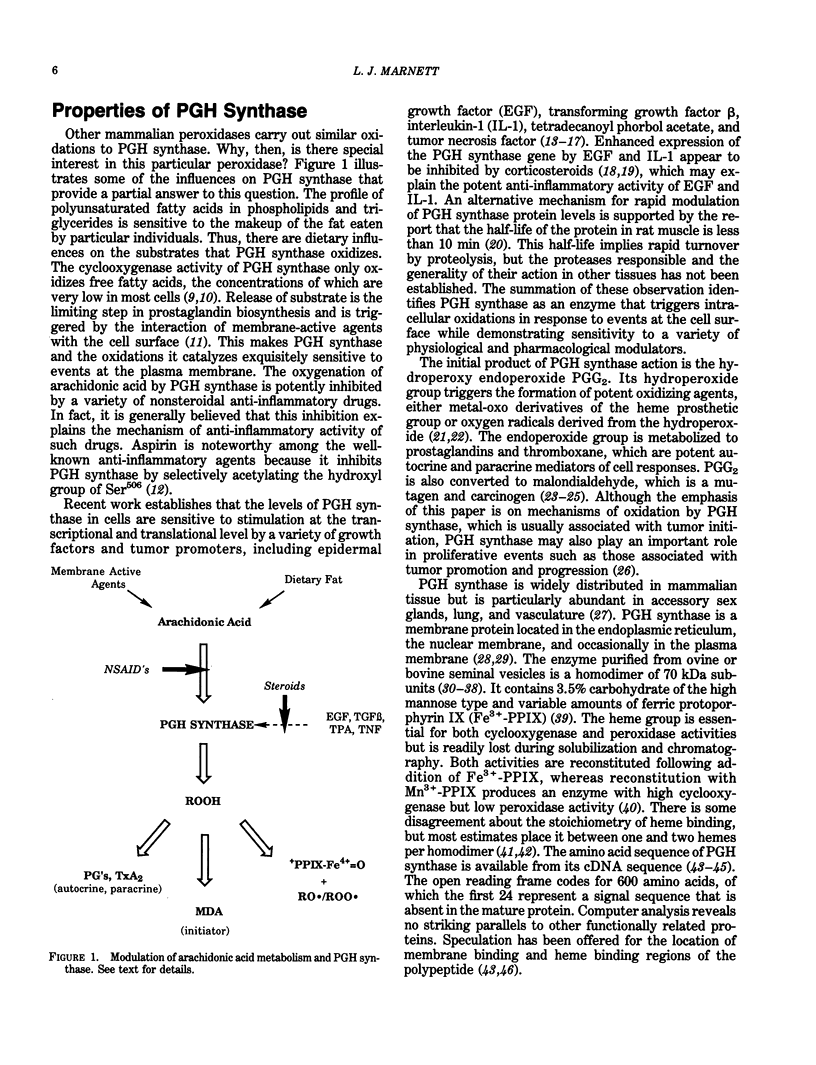
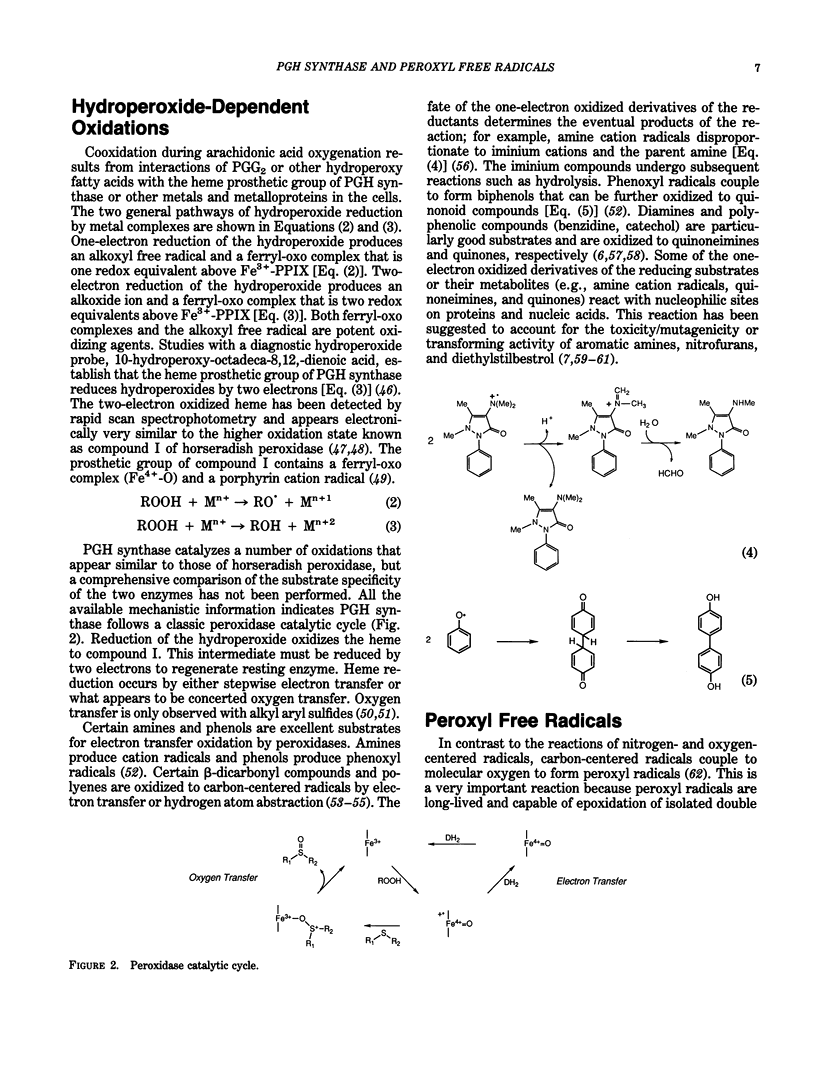
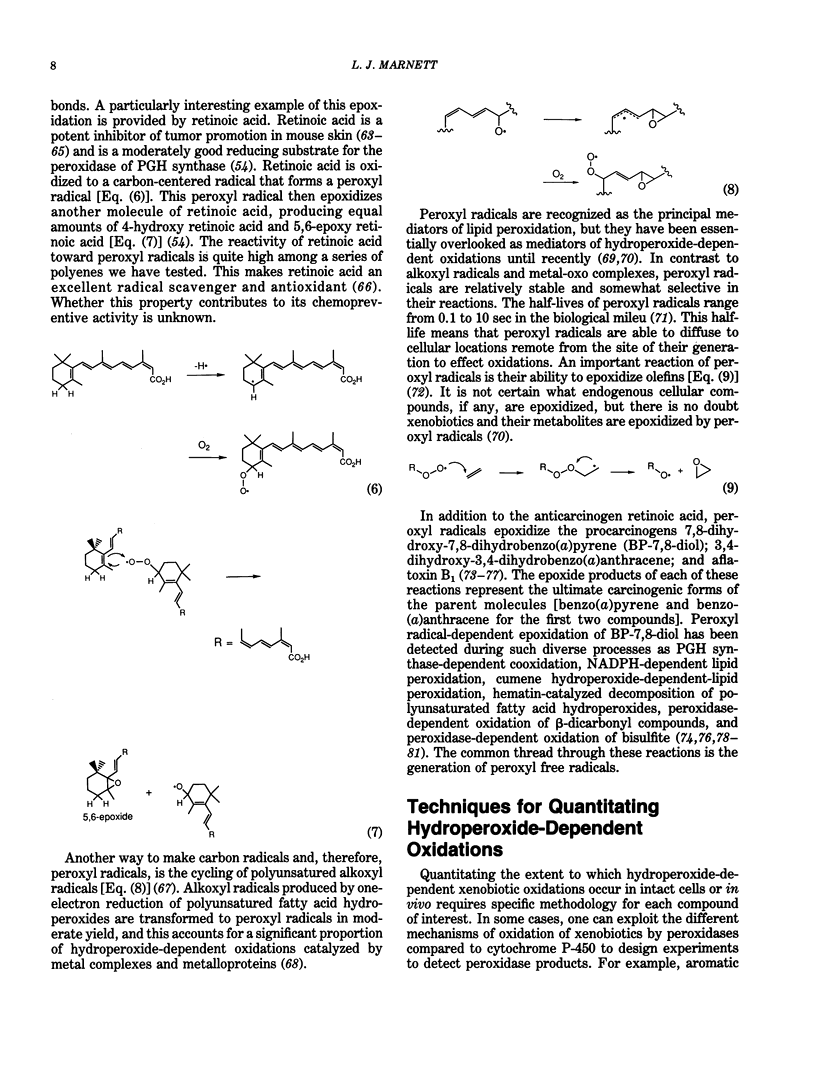
Prostaglandin-H synthase is unique among enzymes of the plant and animal kingdom in its ability to biosynthesize and metabolize hydroperoxides. Its cyclooxygenase activity oxygenates polyunsaturated fatty acids to hydroperoxy endoperoxides, and its peroxidase activity reduces the hydroperoxy group to hydroxy groups. Higher oxidation states of the peroxidase oxidize reducing substrates to electron-deficient derivatives that react with macromolecular nucleophiles. In the case of aromatic amines, the electron-deficient derivatives are mutagenic to bacterial and mammalian cells. beta-Dicarbonyl compounds and retinoic acid are oxidized to carbon-centered radicals that react with O2 to form peroxyl free radicals. Peroxyl radicals are the most stable oxy radicals and are able to diffuse some distance from the site of their generation. Peroxyl radicals are also formed during lipid peroxidation and in the reaction of polyunsaturated fatty acid hydroperoxides with metal complexes and metalloproteins. Peroxyl radicals epoxidize isolated doubled bonds of compounds such as 7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene (BP-7,8-diol); 3,4-dihydroxy-3,4-dihydrobenzo(a)anthracene; and aflatoxin B1. The epoxide products represent the ultimate carcinogenic forms of the respective compounds. Techniques for quantitating the extent of peroxidase dependent or peroxyl radical-dependent metabolism in vivo make use of differences in the structure or stereochemistry of reactive intermediates formed by peroxidases relative to cytochromes P-450. Differences in the relative amounts of hydrolysis products and DNA adducts derived from anti- and syn-dihydrodiolepoxides following application of BP-7,8-diol to mouse skin in vivo indicate peroxyl radicals play a significant role in metabolism of BP-7,8-diol in uninduced animals.
Full text
PDF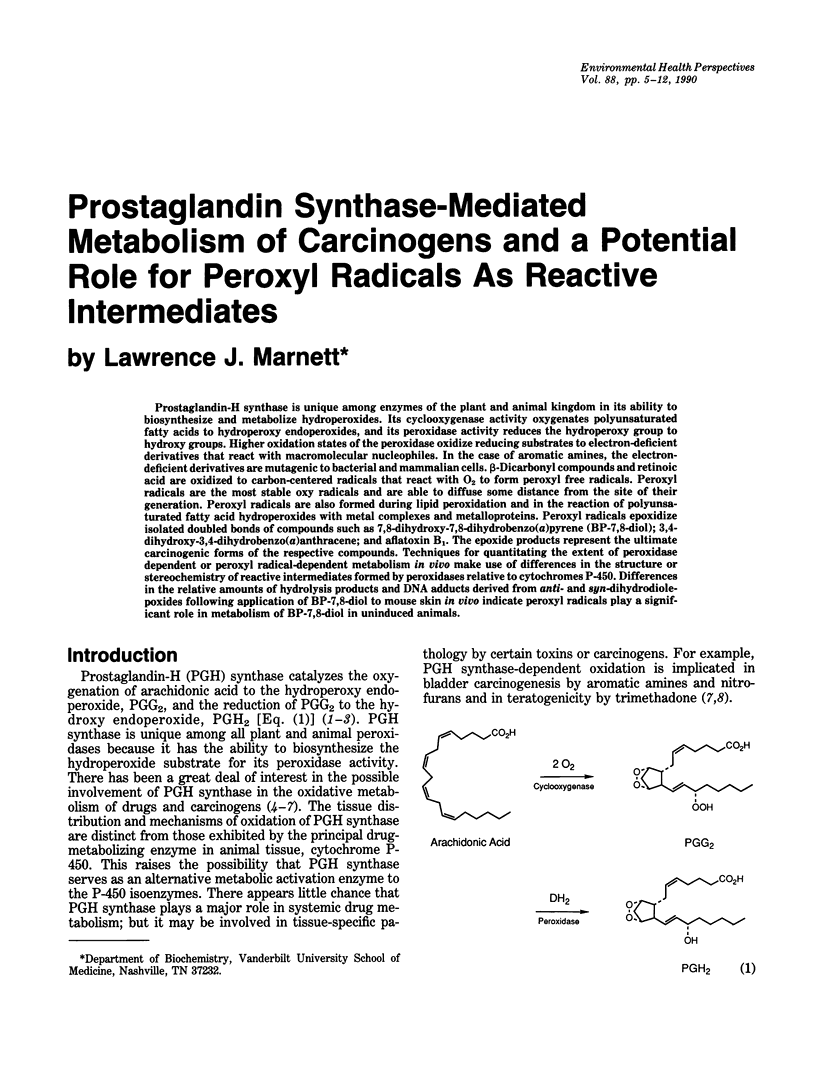



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Makheja A. N., Pash J., Verma M. Corticosteroids suppress cyclooxygenase messenger RNA levels and prostanoid synthesis in cultured vascular cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1159–1163. doi: 10.1016/s0006-291x(88)80995-1. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Muza B., Hla T., Salata K. Restoration of prostacyclin synthase in vascular smooth muscle cells after aspirin treatment: regulation by epidermal growth factor. J Lipid Res. 1985 Jan;26(1):54–61. [PubMed] [Google Scholar]
- Basu A. K., Marnett L. J. Unequivocal demonstration that malondialdehyde is a mutagen. Carcinogenesis. 1983;4(3):331–333. doi: 10.1093/carcin/4.3.331. [DOI] [PubMed] [Google Scholar]
- Battista J. R., Marnett L. J. Prostaglandin H synthase-dependent epoxidation of aflatoxin B1. Carcinogenesis. 1985 Aug;6(8):1227–1229. doi: 10.1093/carcin/6.8.1227. [DOI] [PubMed] [Google Scholar]
- Bollag W. Prophylaxis of chemically induced benign and malignant epithelial tumors by vitamin A acid (retinoic acid). Eur J Cancer. 1972 Dec;8(6):689–693. doi: 10.1016/0014-2964(72)90153-3. [DOI] [PubMed] [Google Scholar]
- Bull A. W. Reducing substrate activity of some aromatic amines for prostaglandin H synthase. Carcinogenesis. 1987 Mar;8(3):387–390. doi: 10.1093/carcin/8.3.387. [DOI] [PubMed] [Google Scholar]
- Casey M. L., Korte K., MacDonald P. C. Epidermal growth factor stimulation of prostaglandin E2 biosynthesis in amnion cells. Induction of prostaglandin H2 synthase. J Biol Chem. 1988 Jun 5;263(16):7846–7854. [PubMed] [Google Scholar]
- DeWitt D. L., Smith W. L. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1412–1416. doi: 10.1073/pnas.85.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen G. H., Wong A., Eling T. E., Barrett J. C., McLachlan J. A. Involvement of prostaglandin synthetase in the peroxidative metabolism of diethylstilbestrol in Syrian hamster embryo fibroblast cell cultures. Cancer Res. 1983 Mar;43(3):992–996. [PubMed] [Google Scholar]
- Dix T. A., Buck J. R., Marnett L. J. Hydroperoxide-dependent epoxidation of 3,4-dihydroxy-3,4-dihydrobenzo[a]anthracene by ram seminal vesicle microsomes and by hematin. Biochem Biophys Res Commun. 1986 Oct 15;140(1):181–187. doi: 10.1016/0006-291x(86)91074-0. [DOI] [PubMed] [Google Scholar]
- Dix T. A., Fontana R., Panthani A., Marnett L. J. Hematin-catalyzed epoxidation of 7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by polyunsaturated fatty acid hydroperoxides. J Biol Chem. 1985 May 10;260(9):5358–5365. [PubMed] [Google Scholar]
- Dix T. A., Marnett L. J. Conversion of linoleic acid hydroperoxide to hydroxy, keto, epoxyhydroxy, and trihydroxy fatty acids by hematin. J Biol Chem. 1985 May 10;260(9):5351–5357. [PubMed] [Google Scholar]
- Dix T. A., Marnett L. J. Detection of the metabolism of polycyclic aromatic hydrocarbon derivatives to ultimate carcinogens during lipid peroxidation. Methods Enzymol. 1984;105:347–352. doi: 10.1016/s0076-6879(84)05046-1. [DOI] [PubMed] [Google Scholar]
- Dix T. A., Marnett L. J. Metabolism of polycyclic aromatic hydrocarbon derivatives to ultimate carcinogens during lipid peroxidation. Science. 1983 Jul 1;221(4605):77–79. doi: 10.1126/science.6304879. [DOI] [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., Baptista E. M., Kennicott K. L., VandenHeuvel W. J., Walker R. W., Fagerness P. E., Kuehl F. A., Jr Oxidation reactions by prostaglandin cyclooxygenase-hydroperoxidase. J Biol Chem. 1981 Jul 25;256(14):7352–7361. [PubMed] [Google Scholar]
- Egan R. W., Gale P. H., VandenHeuvel W. J., Baptista E. M., Kuehl F. A., Jr Mechanism of oxygen transfer by prostaglandin hydroperoxidase. J Biol Chem. 1980 Jan 25;255(2):323–326. [PubMed] [Google Scholar]
- Eling T. E., Mason R. P., Sivarajah K. The formation of aminopyrine cation radical by the peroxidase activity of prostaglandin H synthase and subsequent reactions of the radical. J Biol Chem. 1985 Feb 10;260(3):1601–1607. [PubMed] [Google Scholar]
- Eling T., Curtis J., Battista J., Marnett L. J. Oxidation of (+)-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by mouse keratinocytes: evidence for peroxyl radical- and monoxygenase-dependent metabolism. Carcinogenesis. 1986 Dec;7(12):1957–1963. doi: 10.1093/carcin/7.12.1957. [DOI] [PubMed] [Google Scholar]
- Fagan J. M., Goldberg A. L. Inhibitors of protein and RNA synthesis cause a rapid block in prostaglandin production at the prostaglandin synthase step. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2771–2775. doi: 10.1073/pnas.83.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürstenberger G., Gross M., Marks F. Eicosanoids and multistage carcinogenesis in NMRI mouse skin: role of prostaglandins E and F in conversion (first stage of tumor promotion) and promotion (second stage of tumor promotion). Carcinogenesis. 1989 Jan;10(1):91–96. doi: 10.1093/carcin/10.1.91. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J Biol Chem. 1967 Nov 25;242(22):5344–5354. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M., Lands W. E. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J Biol Chem. 1976 Sep 25;251(18):5575–5579. [PubMed] [Google Scholar]
- Hughes M. F., Mason R. P., Eling T. E. Prostaglandin hydroperoxidase-dependent oxidation of phenylbutazone: relationship to inhibition of prostaglandin cyclooxygenase. Mol Pharmacol. 1988 Aug;34(2):186–193. [PubMed] [Google Scholar]
- Josephy P. D., Iwaniw D. C. Identification of the N-acetylcysteine conjugate of benzidine formed in the peroxidase activation system. Carcinogenesis. 1985 Jan;6(1):155–158. doi: 10.1093/carcin/6.1.155. [DOI] [PubMed] [Google Scholar]
- Karthein R., Dietz R., Nastainczyk W., Ruf H. H. Higher oxidation states of prostaglandin H synthase. EPR study of a transient tyrosyl radical in the enzyme during the peroxidase reaction. Eur J Biochem. 1988 Jan 15;171(1-2):313–320. doi: 10.1111/j.1432-1033.1988.tb13792.x. [DOI] [PubMed] [Google Scholar]
- Krauss R. S., Angerman-Stewart J., Eling T. E., Dooley K. L., Kadlubar F. F. The formation of 2-aminofluorene-DNA adducts in vivo: evidence for peroxidase-mediated activation. J Biochem Toxicol. 1989 Summer;4(2):111–117. doi: 10.1002/jbt.2570040207. [DOI] [PubMed] [Google Scholar]
- Krauss R. S., Eling T. E. Formation of unique arylamine:DNA adducts from 2-aminofluorene activated by prostaglandin H synthase. Cancer Res. 1985 Apr;45(4):1680–1686. [PubMed] [Google Scholar]
- Kulmacz R. J., Lands W. E. Prostaglandin H synthase. Stoichiometry of heme cofactor. J Biol Chem. 1984 May 25;259(10):6358–6363. [PubMed] [Google Scholar]
- Lambeir A. M., Markey C. M., Dunford H. B., Marnett L. J. Spectral properties of the higher oxidation states of prostaglandin H synthase. J Biol Chem. 1985 Dec 5;260(28):14894–14896. [PubMed] [Google Scholar]
- Lands W. E., Samuelsson B. Phospholipid precursors of prostaglandins. Biochim Biophys Acta. 1968 Oct 22;164(2):426–429. doi: 10.1016/0005-2760(68)90168-9. [DOI] [PubMed] [Google Scholar]
- Markey C. M., Alward A., Weller P. E., Marnett L. J. Quantitative studies of hydroperoxide reduction by prostaglandin H synthase. Reducing substrate specificity and the relationship of peroxidase to cyclooxygenase activities. J Biol Chem. 1987 May 5;262(13):6266–6279. [PubMed] [Google Scholar]
- Marnett L. J., Bienkowski M. J., Pagels W. R., Reed G. A. Mechanism of xenobiotic cooxygenation coupled to prostaglandin H2 biosynthesis. Adv Prostaglandin Thromboxane Res. 1980;6:149–151. [PubMed] [Google Scholar]
- Marnett L. J., Chen Y. N., Maddipati K. R., Plé P., Labèque R. Functional differentiation of cyclooxygenase and peroxidase activities of prostaglandin synthase by trypsin treatment. Possible location of a prosthetic heme binding site. J Biol Chem. 1988 Nov 15;263(32):16532–16535. [PubMed] [Google Scholar]
- Marnett L. J., Johnson J. T., Bienkowski M. J. Arachidonic acid-dependent metabolism of 7,8-dihydroxy-7,8-dihydro-benzo[a]pyrene by ram seminal vesicles. FEBS Lett. 1979 Oct 1;106(1):13–16. doi: 10.1016/0014-5793(79)80684-5. [DOI] [PubMed] [Google Scholar]
- Marnett L. J. Peroxyl free radicals: potential mediators of tumor initiation and promotion. Carcinogenesis. 1987 Oct;8(10):1365–1373. doi: 10.1093/carcin/8.10.1365. [DOI] [PubMed] [Google Scholar]
- Marnett L. J., Reed G. A., Dennison D. J. Prostaglandin synthetase dependent activation of 7,8-dihydro-7,8-dihydroxy-geno (a) pyrene to mutagenic derivativies. Biochem Biophys Res Commun. 1978 May 15;82(1):210–216. doi: 10.1016/0006-291x(78)90597-1. [DOI] [PubMed] [Google Scholar]
- Marnett L. J., Siedlik P. H., Ochs R. C., Pagels W. R., Das M., Honn K. V., Warnock R. H., Tainer B. E., Eling T. E. Mechanism of the stimulation of prostaglandin H synthase and prostacyclin synthase by the antithrombotic and antimetastatic agent, nafazatrom. Mol Pharmacol. 1984 Sep;26(2):328–335. [PubMed] [Google Scholar]
- Marnett L. J., Wlodawer P., Samuelsson B. Co-oxygenation of organic substrates by the prostaglandin synthetase of sheep vesicular gland. J Biol Chem. 1975 Nov 10;250(21):8510–8517. [PubMed] [Google Scholar]
- Melikian A. A., Bagheri K., Hecht S. S. Contrasting disposition and metabolism of topically applied benzo(a)pyrene, trans-7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene, and 7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene in mouse epidermis in vivo. Cancer Res. 1987 Oct 15;47(20):5354–5360. [PubMed] [Google Scholar]
- Merlie J. P., Fagan D., Mudd J., Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988 Mar 15;263(8):3550–3553. [PubMed] [Google Scholar]
- Mevkh A. T., Sud'ina G. F., Golub N. B., Varfolomeev S. D. Purification of prostaglandin H synthetase and a fluorometric assay for its activity. Anal Biochem. 1985 Oct;150(1):91–96. doi: 10.1016/0003-2697(85)90444-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto T., Ogino N., Yamamoto S., Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1976 May 10;251(9):2629–2636. [PubMed] [Google Scholar]
- Mutsaers J. H., van Halbeek H., Kamerling J. P., Vliegenthart J. F. Determination of the structure of the carbohydrate chains of prostaglandin endoperoxide synthase from sheep. Eur J Biochem. 1985 Mar 15;147(3):569–574. doi: 10.1111/j.0014-2956.1985.00569.x. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H., Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta. 1973 Dec 20;326(3):448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- Ogino N., Ohki S., Yamamoto S., Hayaishi O. Prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. Inactivation and activation by heme and other metalloporphyrins. J Biol Chem. 1978 Jul 25;253(14):5061–5068. [PubMed] [Google Scholar]
- Ohki S., Ogino N., Yamamoto S., Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979 Feb 10;254(3):829–836. [PubMed] [Google Scholar]
- Panthananickal A., Marnett L. J. Arachidonic acid-dependent metabolism of (+/-)-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene to polyguanylic acid-binding derivatives. Chem Biol Interact. 1981 Jan;33(2-3):239–252. doi: 10.1016/0009-2797(81)90044-2. [DOI] [PubMed] [Google Scholar]
- Pruess-Schwartz D., Nimesheim A., Marnett L. J. Peroxyl radical- and cytochrome P-450-dependent metabolic activation of (+)-7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene in mouse skin in vitro and in vivo. Cancer Res. 1989 Apr 1;49(7):1732–1737. [PubMed] [Google Scholar]
- Pryor W. A. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- Raz A., Wyche A., Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. doi: 10.1073/pnas.86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Wyche A., Siegel N., Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [PubMed] [Google Scholar]
- Reed G. A., Brooks E. A., Eling T. E. Phenylbutazone-dependent epoxidation of 7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene. A new mechanism for prostaglandin H synthase-catalyzed oxidations. J Biol Chem. 1984 May 10;259(9):5591–5595. [PubMed] [Google Scholar]
- Reed G. A., Curtis J. F., Mottley C., Eling T. E., Mason R. P. Epoxidation of (+/-)-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene during (bi)sulfite autoxidation: activation of a procarcinogen by a cocarcinogen. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7499–7502. doi: 10.1073/pnas.83.19.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed G. A., Grafstrom R. C., Krauss R. S., Autrup H., Eling T. E. Prostaglandin H synthase-dependent co-oxygenation of (+/-)-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene in hamster trachea and human bronchus explants. Carcinogenesis. 1984 Jul;5(7):955–960. doi: 10.1093/carcin/5.7.955. [DOI] [PubMed] [Google Scholar]
- Robertson I. G., Sivarajah K., Eling T. E., Zeiger E. Activation of some aromatic amines to mutagenic products by prostaglandin endoperoxide synthetase. Cancer Res. 1983 Feb;43(2):476–480. [PubMed] [Google Scholar]
- Rollins T. E., Smith W. L. Subcellular localization of prostaglandin-forming cyclooxygenase in Swiss mouse 3T3 fibroblasts by electron microscopic immunocytochemistry. J Biol Chem. 1980 May 25;255(10):4872–4875. [PubMed] [Google Scholar]
- Roth G. J., Machuga E. T., Ozols J. Isolation and covalent structure of the aspirin-modified, active-site region of prostaglandin synthetase. Biochemistry. 1983 Sep 27;22(20):4672–4675. doi: 10.1021/bi00289a010. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Machuga E. T., Strittmatter P. The heme-binding properties of prostaglandin synthetase from sheep vesicular gland. J Biol Chem. 1981 Oct 10;256(19):10018–10022. [PubMed] [Google Scholar]
- Ruf H. H., Schuhn D., Nastainczyk W. EPR titration of ovine prostaglandin H synthase with hemin. FEBS Lett. 1984 Jan 9;165(2):293–296. doi: 10.1016/0014-5793(84)80189-1. [DOI] [PubMed] [Google Scholar]
- Samokyszyn V. M., Marnett L. J. Hydroperoxide-dependent cooxidation of 13-cis-retinoic acid by prostaglandin H synthase. J Biol Chem. 1987 Oct 15;262(29):14119–14133. [PubMed] [Google Scholar]
- Sivarajah K., Mukhtar H., Eling T. Arachidonic acid-dependent metabolism of (+/-) trans-7,8-dihydroxy-7,8-dihydro-benzo[a]pyrene (BP-7,8 DIOL) to 7,10/8,9 tetrols. FEBS Lett. 1979 Oct 1;106(1):17–20. doi: 10.1016/0014-5793(79)80685-7. [DOI] [PubMed] [Google Scholar]
- Smith W. L., DeWitt D. L., Allen M. L. Bimodal distribution of the prostaglandin I2 synthase antigen in smooth muscle cells. J Biol Chem. 1983 May 10;258(9):5922–5926. [PubMed] [Google Scholar]
- Van der Ouderaa F. J., Buytenhek M., Nugteren D. H., Van Dorp D. A. Purification and characterisation of prostaglandin endoperoxide synthetase from sheep vesicular glands. Biochim Biophys Acta. 1977 May 25;487(2):315–331. doi: 10.1016/0005-2760(77)90008-x. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Rice H. M., Shapas B. G., Boutwell R. K. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase activity in mouse epidermis by vitamin A analogs (retinoids). Cancer Res. 1978 Mar;38(3):793–801. [PubMed] [Google Scholar]
- Vonkeman H., van Dorp D. A. The action of prostaglandin synthetase on 2-arachidonyl-lecithin. Biochim Biophys Acta. 1968 Oct 22;164(2):430–432. doi: 10.1016/0005-2760(68)90169-0. [DOI] [PubMed] [Google Scholar]
- Wells P. G., Nagai M. K., Greco G. S. Inhibition of trimethadione and dimethadione teratogenicity by the cyclooxygenase inhibitor acetylsalicylic acid: a unifying hypothesis for the teratologic effects of hydantoin anticonvulsants and structurally related compounds. Toxicol Appl Pharmacol. 1989 Mar 1;97(3):406–414. doi: 10.1016/0041-008x(89)90245-7. [DOI] [PubMed] [Google Scholar]
- Wild D., Degen G. H. Prostaglandin H synthase-dependent mutagenic activation of heterocyclic aromatic amines of the IQ-type. Carcinogenesis. 1987 Apr;8(4):541–545. doi: 10.1093/carcin/8.4.541. [DOI] [PubMed] [Google Scholar]
- Wu K. K., Hatzakis H., Lo S. S., Seong D. C., Sanduja S. K., Tai H. H. Stimulation of de novo synthesis of prostaglandin G/H synthase in human endothelial cells by phorbol ester. J Biol Chem. 1988 Dec 15;263(35):19043–19047. [PubMed] [Google Scholar]
- Yamamoto S. Purification and assay of PGH synthase from bovine seminal vesicles. Methods Enzymol. 1982;86:55–60. doi: 10.1016/0076-6879(82)86168-5. [DOI] [PubMed] [Google Scholar]
- Yokoyama C., Takai T., Tanabe T. Primary structure of sheep prostaglandin endoperoxide synthase deduced from cDNA sequence. FEBS Lett. 1988 Apr 25;231(2):347–351. doi: 10.1016/0014-5793(88)80847-0. [DOI] [PubMed] [Google Scholar]
- van der Ouderaa F. J., Buytenhek M. Purification of PGH synthase from sheep vesicular glands. Methods Enzymol. 1982;86:60–68. doi: 10.1016/0076-6879(82)86169-7. [DOI] [PubMed] [Google Scholar]



