Abstract
alpha, beta-Unsaturated carbonyl compounds are important not only from a theoretical but also a practical standpoint. These ubiquitous compounds can interact with DNA through various mechanisms. The predominant interaction is the formation of cyclic 1,N2-deoxyguanosine adducts; 7,8-cyclic guanine adducts are also found. We have synthesized and characterized the stereoisomers of adducts formed by about 20 alpha, beta-unsaturated carbonyl compounds. The different types of adducts and the mutagenic and genotoxic response can be explained by the molecular structures of the agents. Compounds forming saturated cyclic adducts are mutagenic in S. typhimurium strain TA100 and to a lesser extent in TA1535. Substances with a leaving group at the C-3 position form unsaturated conjugated cyclic adducts and are mutagenic only in the His D3052 frameshift strains with an intact excision repair system (no urvA mutation). Metabolic epoxidation of the double bond and other metabolic activation, e.g., activation of the nitrogroups via nitroreductases, were also found to contribute to genotoxic and mutagenic activities. Our results have further elucidated the genotoxic mechanisms of these compounds; however, additional investigations are required for a complete understanding of the genotoxic activity of this class of compounds.
Full text
PDF
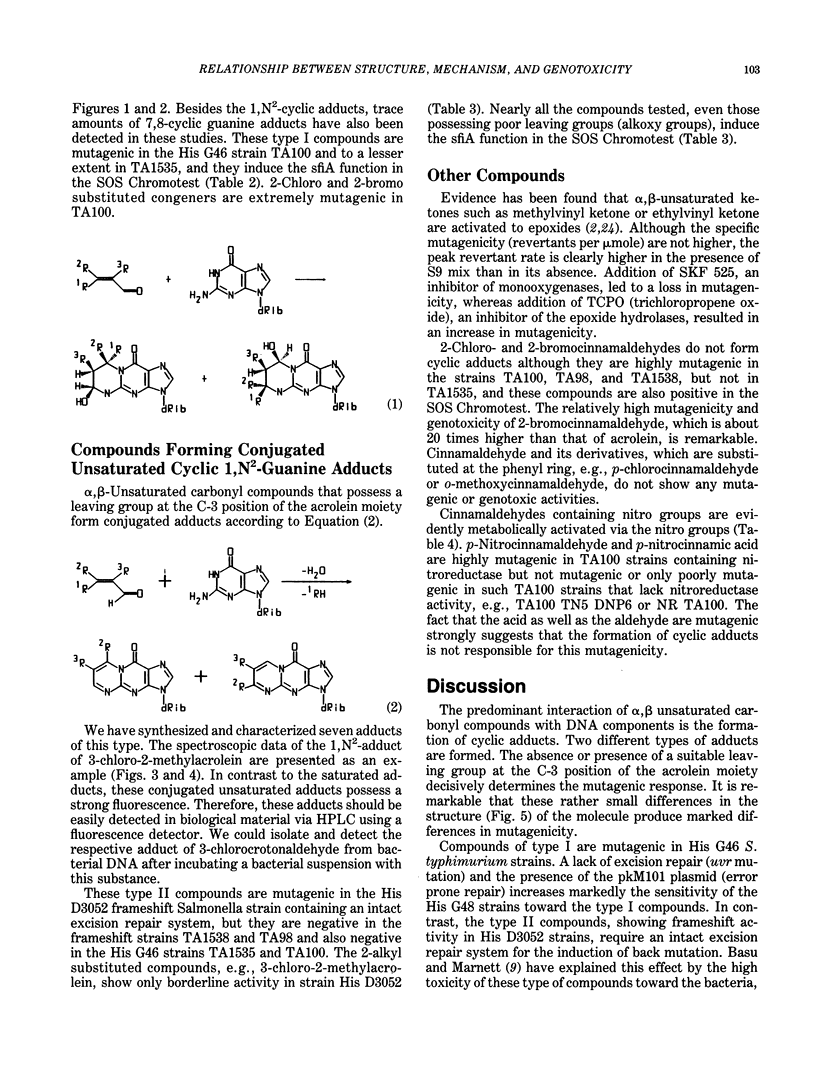
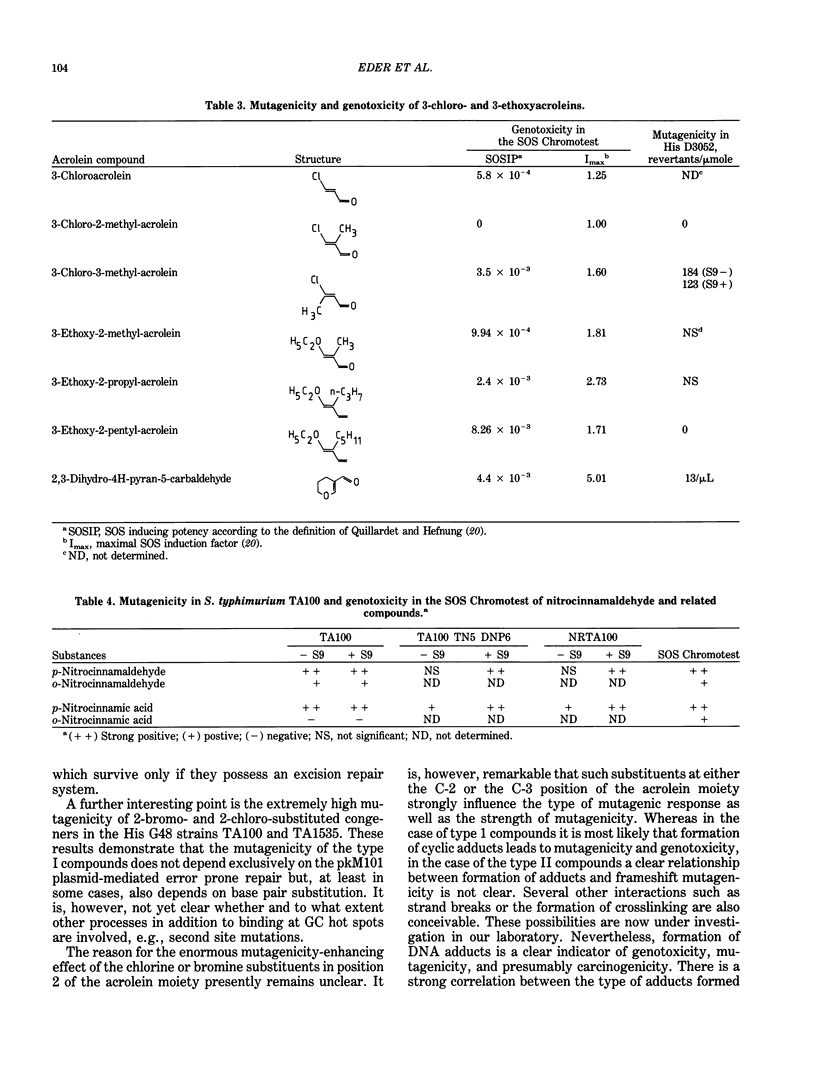


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu A. K., Marnett L. J. Molecular requirements for the mutagenicity of malondialdehyde and related acroleins. Cancer Res. 1984 Jul;44(7):2848–2854. [PubMed] [Google Scholar]
- Basu A. K., O'Hara S. M., Valladier P., Stone K., Mols O., Marnett L. J. Identification of adducts formed by reaction of guanine nucleosides with malondialdehyde and structurally related aldehydes. Chem Res Toxicol. 1988 Jan-Feb;1(1):53–59. doi: 10.1021/tx00001a010. [DOI] [PubMed] [Google Scholar]
- Chung F. L., Tanaka T., Hecht S. S. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986 Mar;46(3):1285–1289. [PubMed] [Google Scholar]
- Chung F. L., Young R., Hecht S. S. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984 Mar;44(3):990–995. [PubMed] [Google Scholar]
- Draminski W., Eder E., Henschler D. A new pathway of acrolein metabolism in rats. Arch Toxicol. 1983 Mar;52(3):243–247. doi: 10.1007/BF00333903. [DOI] [PubMed] [Google Scholar]
- Eder E., Deininger C., Kütt W. Genotoxicity of monofunctional methanesulphonates in the SOS chromotest as a function of alkylation mechanisms. A comparison with the mutagenicity in S. typhimurium TA100. Mutat Res. 1989 Mar;211(1):51–64. doi: 10.1016/0027-5107(89)90106-1. [DOI] [PubMed] [Google Scholar]
- Eder E., Dornbusch K. Metabolism of 2,3-dichloro-1-propene in the rat. Consideration of bioactivation mechanisms. Drug Metab Dispos. 1988 Jan-Feb;16(1):60–68. [PubMed] [Google Scholar]
- Eder E., Favre A., Deininger C., Hahn H., Kütt W. Induction of SOS repair by monofunctional methanesulphonates in various Escherichia coli strains. Structure-activity relationships in comparison with mutagenicity in Salmonella typhimurium. Mutagenesis. 1989 May;4(3):179–186. doi: 10.1093/mutage/4.3.179. [DOI] [PubMed] [Google Scholar]
- Eder E., Henschler D., Neudecker T. Mutagenic properties of allylic and alpha, beta-unsaturated compounds: consideration of alkylating mechanisms. Xenobiotica. 1982 Dec;12(12):831–848. doi: 10.3109/00498258209038955. [DOI] [PubMed] [Google Scholar]
- Eder E., Neudecker T., Lutz D., Henschler D. Mutagenic potential of allyl and allylic compounds. Structure-activity relationship as determined by alkylating and direct in vitro mutagenic properties. Biochem Pharmacol. 1980 Apr 1;29(7):993–998. doi: 10.1016/0006-2952(80)90161-6. [DOI] [PubMed] [Google Scholar]
- Haworth S., Lawlor T., Mortelmans K., Speck W., Zeiger E. Salmonella mutagenicity test results for 250 chemicals. Environ Mutagen. 1983;5 (Suppl 1):1–142. [PubMed] [Google Scholar]
- Hoffman C., Bastian H., Wiedenmann M., Deininger C., Eder E. Detection of acrolein congener-DNA adducts isolated from cellular systems. Arch Toxicol Suppl. 1989;13:219–223. doi: 10.1007/978-3-642-74117-3_34. [DOI] [PubMed] [Google Scholar]
- Marnett L. J., Hurd H. K., Hollstein M. C., Levin D. E., Esterbauer H., Ames B. N. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat Res. 1985 Jan-Feb;148(1-2):25–34. doi: 10.1016/0027-5107(85)90204-0. [DOI] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Neudecker T., Eder E., Deininger C., Hoffman C., Henschler D. Mutagenicity of methylvinyl ketone in Salmonella typhimurium TA100--indication for epoxidation as an activation mechanism. Mutat Res. 1989 Oct;227(2):131–134. doi: 10.1016/0165-7992(89)90009-2. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Hofnung M. The SOS Chromotest, a colorimetric bacterial assay for genotoxins: procedures. Mutat Res. 1985 Jun;147(3):65–78. doi: 10.1016/0165-1161(85)90020-2. [DOI] [PubMed] [Google Scholar]
- Rannug U., Göthe R., Wachtmeister C. A. The mutagenicity of chloroethylene oxide, chloroacetaldehyde, 2-chloroethanol and chloroacetic acid, conceivable metabolites of vinyl chloride. Chem Biol Interact. 1976 Mar;12(3-4):251–263. doi: 10.1016/0009-2797(76)90041-7. [DOI] [PubMed] [Google Scholar]


