Abstract
Aryl hydrocarbon hydroxylase (AHH)-inducing potency of eight polychlorinated dibenzofuran (PCDF) isomers, 3,4,5,3',4',5'-hexachlorobiphenyl (HCB) and 2,3,7,8-tetrachlorodibenzo-p-dioxon (TCDD) in two inbred mouse strains (AHH responsive and nonresponsive mouse strains) and eight human lymphoblastoid cell lines (four males and four females) was investigated to evaluate their relative toxic potency. In AHH nonresponsive DBA mouse strain, only TCDD induced hepatic AHH activity at a dose of 30 micrograms/kg, while in AHH responsive C57 mouse strain, six PCDF isomers besides TCDD could enhance the enzyme activity significantly. 2,3,7,8-Tetrachlorodibenzofuran (2,3,7,8-TCDF), 1,2,3,7,8-pentachlorodibenzofuran (1,2,3,7,8-PCDF) and 2,3,4,7,8-pentachlorodibenzofuran (2,3,4,7,8-PCDF) showed the highest AHH inducing activity among the PCDF isomers tested. In contrast with the results obtained from the mouse experiments, in human lymphoblastoid cells, 2,3,4,7,8-PCDF, 1,2,3,4,6,7-hexachlorodibenzofuran (1,2,3,4,6,7-HCDF) and 1,2,3,7,8-hexachlorodibenzofuran (1,2,3,4,7,8-HCDF) elicited the highest AHH induction and were as potent AHH inducers as TCDD. These observations suggest that toxicities of 2,3,4,7,8-PCDF, 1,2,3,4,6,7-HCDF and 1,2,3,4,7,8-HCDF in human tissues may be comparable to that of TCDD. It was also observed that in both male and female human cell lines, the degree of AHH inducibilities of these compounds were roughly parallel to that of 3-methylcholanthrene, possibly indicating that genetic susceptibility among human population to the toxic compounds are also present similar to those reported among mouse strains.
Full text
PDF

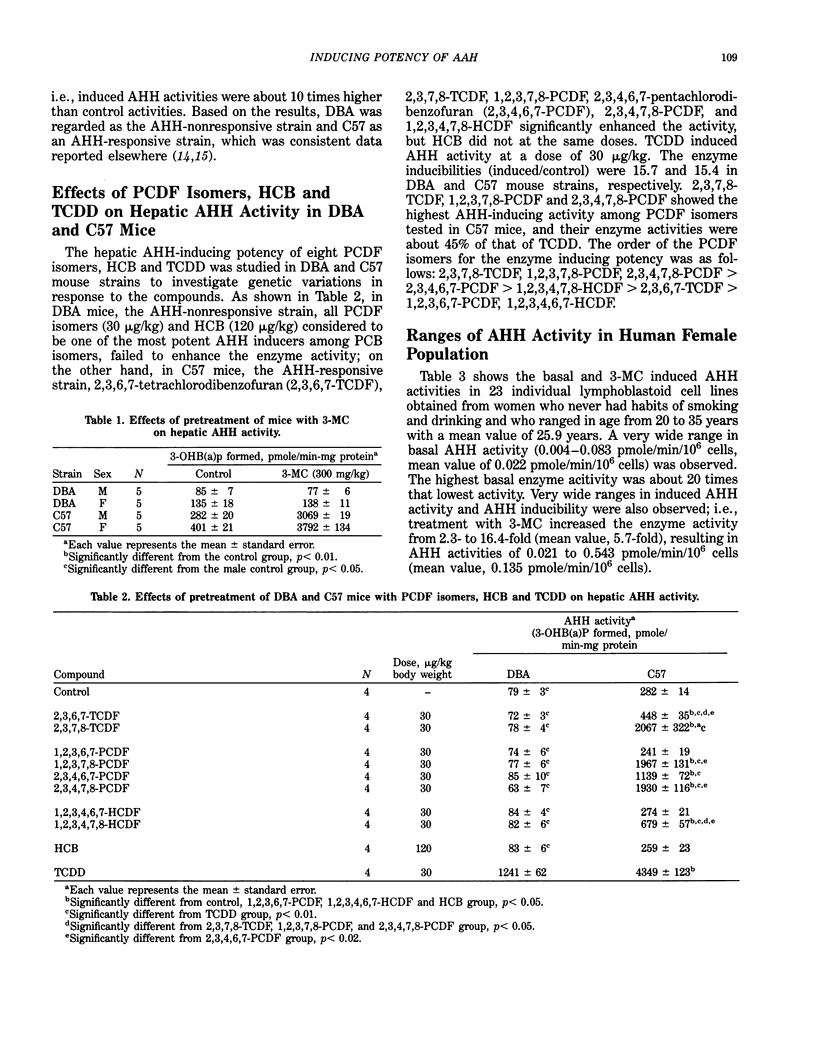

Selected References
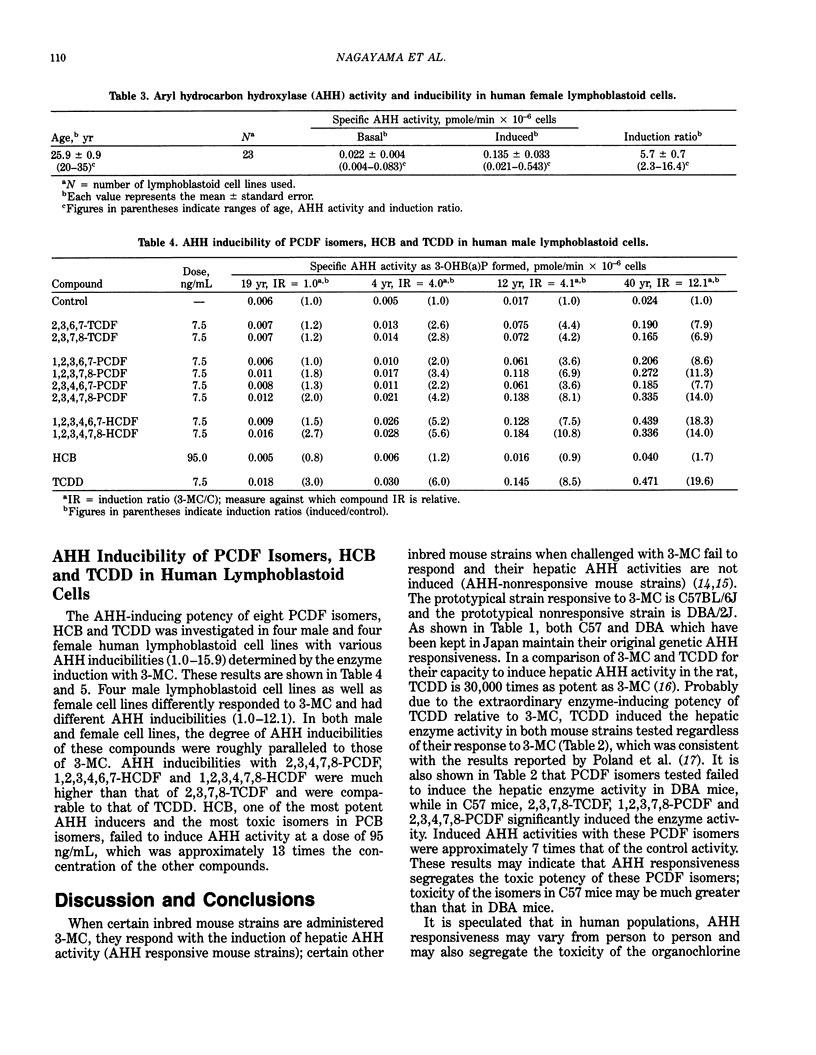
These references are in PubMed. This may not be the complete list of references from this article.
- Bradlaw J. A., Casterline J. L., Jr Induction of enzyme activity in cell culture: a rapid screen for detection of planar polychlorinated organic compounds. J Assoc Off Anal Chem. 1979 Jul;62(4):904–916. [PubMed] [Google Scholar]
- Chen P. H., Wong C. K., Rappe C., Nygren M. Polychlorinated biphenyls, dibenzofurans and quaterphenyls in toxic rice-bran oil and in the blood and tissues of patients with PCB poisoning (Yu-Cheng) in Taiwan. Environ Health Perspect. 1985 Feb;59:59–65. doi: 10.1289/ehp.59-1568079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtoo H. L., Bejba N., Minowada J. Properties, inducibility, and an improved method of analysis of aryl hydrocarbon hydroxylase in cultured human lymphocytes. Cancer Res. 1975 May;35(5):1235–1243. [PubMed] [Google Scholar]
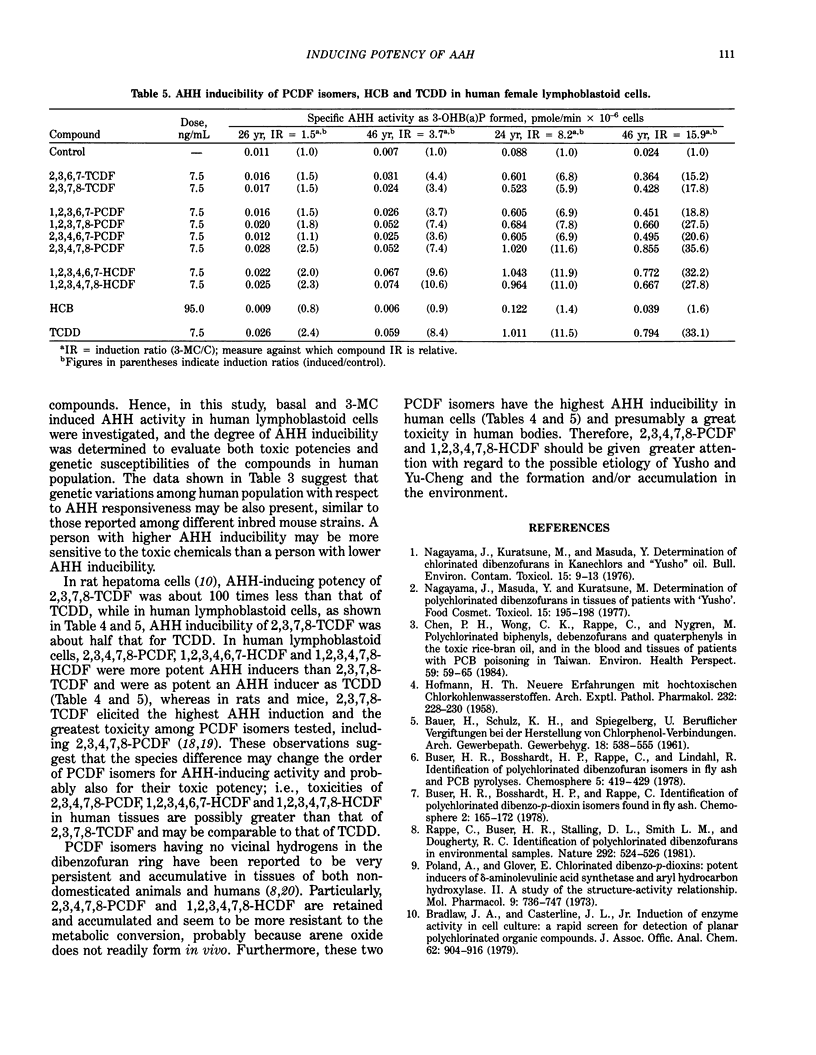
- HOFMANN H. T. Neuere Erfahrungen mit hochtoxischen Chlorkohlenwasserstoffen. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1957;232(1):228–230. [PubMed] [Google Scholar]
- Kodama Y., Bock F. G. Benzo(alpha)pyrene-metabolizing enzyme activity of livers of various strains of mice. Cancer Res. 1970 Jun;30(6):1846–1849. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagayama J., Kuratsune M., Masuda Y. Determination of chlorinated dibenzofurans in kanechlors and "yusho oil". Bull Environ Contam Toxicol. 1976 Jan;15(1):9–13. doi: 10.1007/BF01686189. [DOI] [PubMed] [Google Scholar]
- Nagayama J., Kuroki H., Masuda Y., Kuratsune M. A comparative study of polychlorinated dibenzofurans, polychlorinated biphenyls and 2,3,7,8-tetrachlorodibenzo-p-dioxin on aryl hydrocarbon hydroxylase inducing potency in rats. Arch Toxicol. 1983 Jul;53(3):177–184. doi: 10.1007/BF00316501. [DOI] [PubMed] [Google Scholar]
- Nagayama J., Masuda Y., Kuratsune M. Determination of polychlorinated dibenzofurans in tissues of patients with 'yusho'. Food Cosmet Toxicol. 1977 Jun;15(3):195–198. doi: 10.1016/s0015-6264(77)80389-1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Goujon F. M., Gielen J. E. Aryl hydrocarbon hydroxylase induction by polycyclic hydrocarbons: simple autosomal dominant trait in the mouse. Nat New Biol. 1972 Mar 29;236(65):107–110. doi: 10.1038/newbio236107a0. [DOI] [PubMed] [Google Scholar]
- Poland A. P., Glover E., Robinson J. R., Nebert D. W. Genetic expression of aryl hydrocarbon hydroxylase activity. Induction of monooxygenase activities and cytochrome P1-450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically "nonresponsive" to other aromatic hydrocarbons. J Biol Chem. 1974 Sep 10;249(17):5599–5606. [PubMed] [Google Scholar]
- Poland A., Glover E. 2,3,7,8,-Tetrachlorodibenzo-p-dioxin: segregation of toxocity with the Ah locus. Mol Pharmacol. 1980 Jan;17(1):86–94. [PubMed] [Google Scholar]
- Poland A., Glover E. Chlorinated dibenzo-p-dioxins: potent inducers of delta-aminolevulinic acid synthetase and aryl hydrocarbon hydroxylase. II. A study of the structure-activity relationship. Mol Pharmacol. 1973 Nov;9(6):736–747. [PubMed] [Google Scholar]
- Poland A., Glover E. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin, a potent inducer of aryl hydrocarbon hydroxylase, with 3-methylcholanthrene. Mol Pharmacol. 1974 Mar;10(2):349–359. [PubMed] [Google Scholar]
- Yoshihara S., Nagata K., Yoshimura H., Kuroki H., Masuda Y. Inductive effect on hepatic enzymes and acute toxicity of individual polychlorinated dibenzofuran congeners in rats. Toxicol Appl Pharmacol. 1981 Jul;59(3):580–588. doi: 10.1016/0041-008x(81)90313-6. [DOI] [PubMed] [Google Scholar]


