Abstract
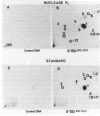
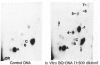
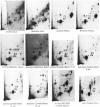
Solid tumors have been reported in the Zymbal gland, oral and nasal cavities, liver, and mammary gland of Sprague-Dawley rats following chronic, high-dose administration of benzene. The carcinogenic activity of benzene is thought to be caused by activation to toxic metabolites that can interact with DNA, forming covalent adducts. A nuclease P1-enhanced 32P-postlabeling assay, having a sensitivity limit of 1 adduct in 10(9-10) DNA nucleotides, was found suitable for measuring aromatic DNA adducts derived in vitro from catechol, benzenetriol (BT), phenol, hydroquinone (HQ), and benzoquinone (BQ), potential metabolites of benzene. When DNA specimens isolated from tissues of female Sprague-Dawley rats at 24 hr after an oral gavage dose of 200 to 500 mg/kg, 5 days/week, in olive oil (3 mL/kg) for 1 day, 1 week, 5 weeks, and 10 weeks were analyzed by the 32P-postlabeling procedure, no aromatic adducts were detected unequivocally with DNA samples of liver, kidney, bone marrow, and mammary gland. With Zymbal gland DNA, three weak spots at levels totaling four lesions per 10(9) DNA nucleotides were seen only after 10 weeks of treatment, and these adducts did not correspond chromatographically to major adducts in vitro from the above specified compounds. Consequently, this finding requires confirmatory experiments. This distinct adduct pattern may relate to tumor induction in this organ following benzene administration. Our results also indicate that DNA adducts derived from catechol, BT, phenol, HQ, and BQ are either not formed in vivo with benzene or formed at levels below the detection limit of 1 adduct per 10(9-10) DNA nucleotides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfellini G., Grilli S., Colacci A., Mazzullo M., Prodi G. In vivo and in vitro binding of benzene to nucleic acids and proteins of various rat and mouse organs. Cancer Lett. 1985 Sep 15;28(2):159–168. doi: 10.1016/0304-3835(85)90071-0. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Kalf G. F. Recent advances in the metabolism and toxicity of benzene. Crit Rev Toxicol. 1987;18(2):141–159. doi: 10.3109/10408448709089859. [DOI] [PubMed] [Google Scholar]
- Latriano L., Goldstein B. D., Witz G. Formation of muconaldehyde, an open-ring metabolite of benzene, in mouse liver microsomes: an additional pathway for toxic metabolites. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8356–8360. doi: 10.1073/pnas.83.21.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L. J., Disher R. M., Reddy M. V., Randerath K. 32P-postlabeling assay in mice of transplacental DNA damage induced by the environmental carcinogens safrole, 4-aminobiphenyl, and benzo(a)pyrene. Cancer Res. 1986 Jun;46(6):3046–3054. [PubMed] [Google Scholar]
- Lutz W. K., Schlatter C. Mechanism of the carcinogenic action of benzene: irreversible binding to rat liver DNA. Chem Biol Interact. 1977 Aug;18(2):241–245. doi: 10.1016/0009-2797(77)90010-2. [DOI] [PubMed] [Google Scholar]
- Maltoni C., Conti B., Cotti G., Belpoggi F. Experimental studies on benzene carcinogenicity at the Bologna Institute of Oncology: current results and ongoing research. Am J Ind Med. 1985;7(5-6):415–446. doi: 10.1002/ajim.4700070508. [DOI] [PubMed] [Google Scholar]
- Marcus W. L. Chemical of current interest--benzene. Toxicol Ind Health. 1987 Mar;3(1):205–266. doi: 10.1177/074823378700300108. [DOI] [PubMed] [Google Scholar]
- Randerath E., Agrawal H. P., Weaver J. A., Bordelon C. B., Randerath K. 32P-postlabeling analysis of DNA adducts persisting for up to 42 weeks in the skin, epidermis and dermis of mice treated topically with 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1985 Aug;6(8):1117–1126. doi: 10.1093/carcin/6.8.1117. [DOI] [PubMed] [Google Scholar]
- Randerath K., Haglund R. E., Phillips D. H., Reddy M. V. 32P-post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. I. Adult female CD-1 mice. Carcinogenesis. 1984 Dec;5(12):1613–1622. doi: 10.1093/carcin/5.12.1613. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Blackburn G. R., Irwin S. E., Kommineni C., Mackerer C. R., Mehlman M. A. A method for in vitro culture of rat Zymbal gland: use in mechanistic studies of benzene carcinogenesis in combination with 32P-postlabeling. Environ Health Perspect. 1989 Jul;82:239–247. doi: 10.1289/ehp.8982239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Gupta R. C., Randerath E., Randerath K. 32P-postlabeling test for covalent DNA binding of chemicals in vivo: application to a variety of aromatic carcinogens and methylating agents. Carcinogenesis. 1984 Feb;5(2):231–243. doi: 10.1093/carcin/5.2.231. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Gupta R. C., Randerath K. 32P-base analysis of DNA. Anal Biochem. 1981 Nov 1;117(2):271–279. doi: 10.1016/0003-2697(81)90722-3. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Irvin T. R., Randerath K. Formation and persistence of sterigmatocystin--DNA adducts in rat liver determined via 32P-postlabeling analysis. Mutat Res. 1985 Oct;152(1):85–96. [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. 32P-analysis of DNA adducts in somatic and reproductive tissues of rats treated with the anticancer antibiotic, mitomycin C. Mutat Res. 1987 Jul;179(1):75–88. doi: 10.1016/0027-5107(87)90043-1. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. 32P-postlabeling assay for carcinogen-DNA adducts: nuclease P1-mediated enhancement of its sensitivity and applications. Environ Health Perspect. 1987 Dec;76:41–47. doi: 10.1289/ehp.877641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Rushmore T., Snyder R., Kalf G. Covalent binding of benzene and its metabolites to DNA in rabbit bone marrow mitochondria in vitro. Chem Biol Interact. 1984 Apr;49(1-2):133–154. doi: 10.1016/0009-2797(84)90057-7. [DOI] [PubMed] [Google Scholar]
- Witz G., Rao G. S., Goldstein B. D. Short-term toxicity of trans,trans-muconaldehyde. Toxicol Appl Pharmacol. 1985 Sep 30;80(3):511–516. doi: 10.1016/0041-008x(85)90396-5. [DOI] [PubMed] [Google Scholar]