Abstract
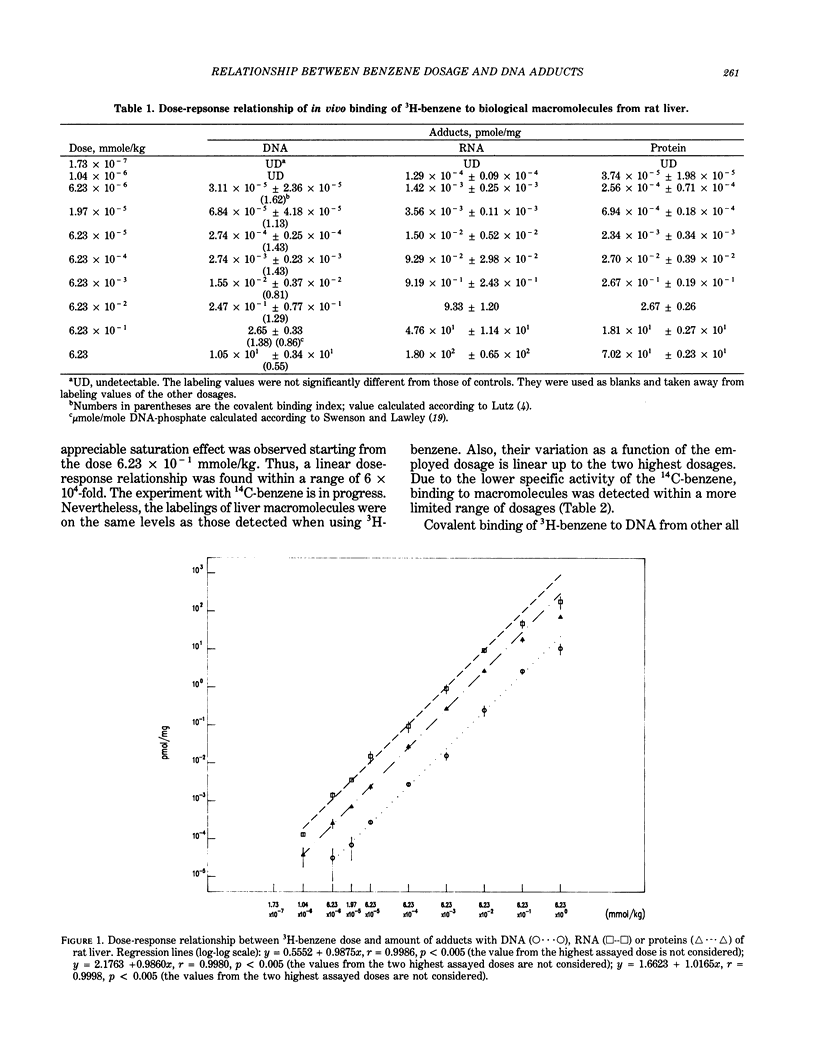
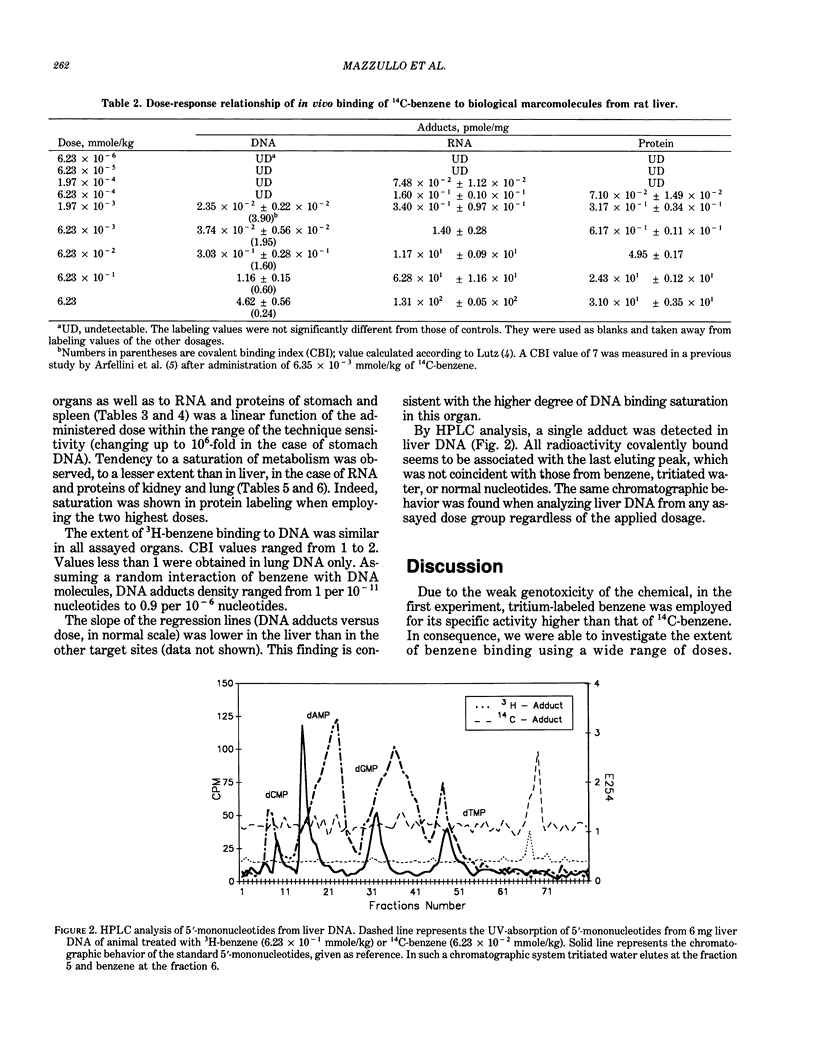
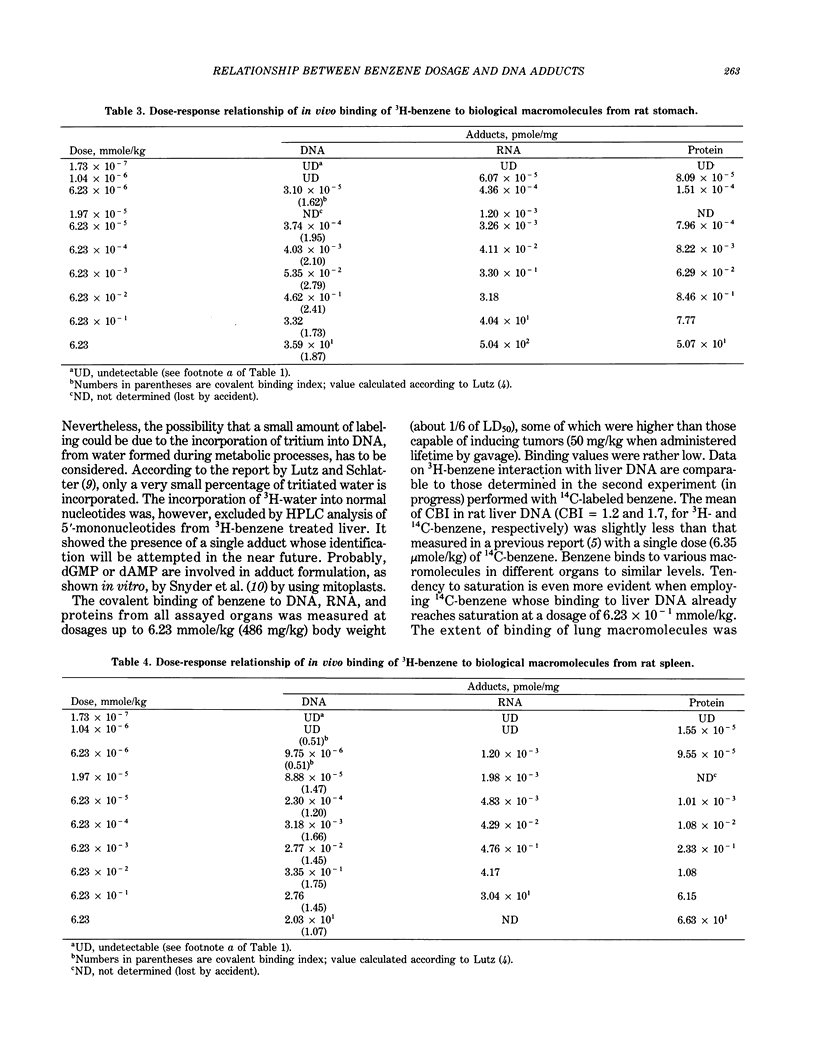
The dose-response relationship of the benzene covalent interaction with biological macromolecules from rat organs was studied. The administered dose range was 3.6 x 10(7) starting from the highest dosage employed, 486 mg/kg, which is oncogenic for rodents, and included low and very low dosages. The present study was initially performed with tritium-labeled benzene, administered by IP injection. In order to exclude the possibility that part of the detected radioactivity was due to tritium incorporated into DNA from metabolic processes, 14C-benzene was then also used following a similar experimental design. By HPLC analysis, a single adduct from benzene-treated DNA was detected; adduct identification will be attempted in the near future. Linear dose-response relationship was observed within most of the range of explored doses. Linearity was particularly evident within low and very low dosages. Saturation of benzene metabolism did occur at the highest dosages for most of the assayed macromolecules and organs, especially in rat liver. This finding could be considered as indicative of the dose-response relationship of tumor induction and could be used in risk assessment.
Full text
PDF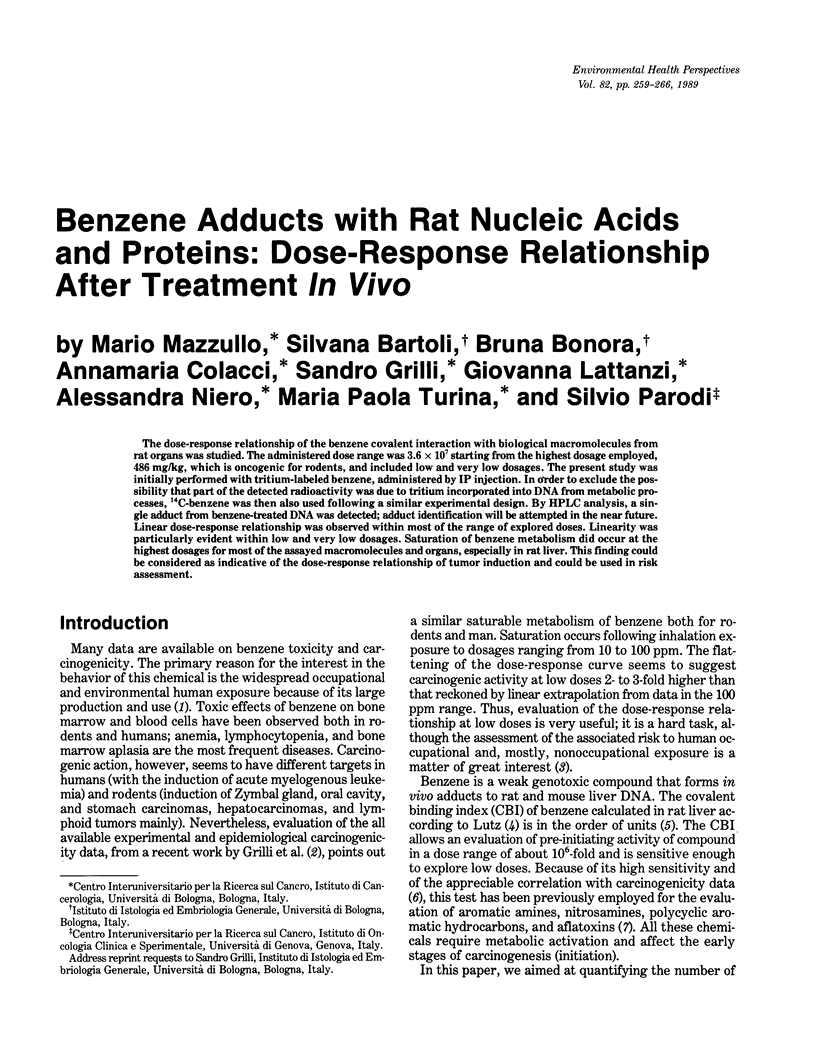



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton B. S., Goetchius M. P., Campbell T. C. Linear dose-response curve for the hepatic macromolecular binding of aflatoxin B1 in rats at very low exposures. Cancer Res. 1982 Sep;42(9):3659–3662. [PubMed] [Google Scholar]
- Arfellini G., Grilli S., Colacci A., Mazzullo M., Prodi G. In vivo and in vitro binding of benzene to nucleic acids and proteins of various rat and mouse organs. Cancer Lett. 1985 Sep 15;28(2):159–168. doi: 10.1016/0304-3835(85)90071-0. [DOI] [PubMed] [Google Scholar]
- Becker R. A., Shank R. C. Kinetics of formation and persistence of ethylguanines in DNA of rats and hamsters treated with diethylnitrosamine. Cancer Res. 1985 May;45(5):2076–2084. [PubMed] [Google Scholar]
- Casanova-Schmitz M., Starr T. B., Heck H. D. Differentiation between metabolic incorporation and covalent binding in the labeling of macromolecules in the rat nasal mucosa and bone marrow by inhaled [14C]- and [3H]formaldehyde. Toxicol Appl Pharmacol. 1984 Oct;76(1):26–44. doi: 10.1016/0041-008x(84)90026-7. [DOI] [PubMed] [Google Scholar]
- Dunn B. P. Wide-range linear dose-response curve for DNA binding of orally administered benzo(a)pyrene in mice. Cancer Res. 1983 Jun;43(6):2654–2658. [PubMed] [Google Scholar]
- Gehring P. J., Watanabe P. G., Park C. N. Resolution of dose-response toxicity data for chemicals requiring metabolic activation: example--vinyl chloride. Toxicol Appl Pharmacol. 1978 Jun;44(3):581–591. doi: 10.1016/0041-008x(78)90266-1. [DOI] [PubMed] [Google Scholar]
- Grilli S., Lutz W. K., Parodi S. Possible implications from results of animal studies in human risk estimations for benzene: nonlinear dose-response relationship due to saturation of metabolism. J Cancer Res Clin Oncol. 1987;113(4):349–358. doi: 10.1007/BF00397718. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Gelboin H. V. Aryl hydrocarbon hydroxylase and polycyclic hydrocarbon tumorigenesis: effect of the enzyme inhibitor 7,8-benzoflavone on tumorigenesis and macromolecule binding. Proc Natl Acad Sci U S A. 1972 Apr;69(4):824–828. doi: 10.1073/pnas.69.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M. S., Anderson M. W. Persistence of benzo(a)pyrene metabolite:DNA adducts in lung and liver of mice. Cancer Res. 1984 Jan;44(1):97–101. [PubMed] [Google Scholar]
- Lutz W. K. Quantitative evaluation of DNA binding data for risk estimation and for classification of direct and indirect carcinogens. J Cancer Res Clin Oncol. 1986;112(2):85–91. doi: 10.1007/BF00404387. [DOI] [PubMed] [Google Scholar]
- Parodi S., Lutz W. K., Colacci A., Mazzullo M., Taningher M., Grilli S. Results of animal studies suggest a nonlinear dose-response relationship for benzene effects. Environ Health Perspect. 1989 Jul;82:171–176. doi: 10.1289/ehp.8982171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi S., Taningher M., Zunino A., Ottaggio L., De Ferrari M., Santi L. Quantitative predictivity of carcinogenicity for sister chromatid exchanges in vivo. Basic Life Sci. 1984;29(Pt A):409–429. doi: 10.1007/978-1-4684-4889-4_32. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Perry W. Alkylation of nucleic acids and metabolism of small doses of dimethylnitrosamine in the rat. Cancer Res. 1981 Aug;41(8):3128–3132. [PubMed] [Google Scholar]
- Pereira M. A., Burns F. J., Albert R. E. Dose response for benzo(a)pyrene adducts in mouse epidermal DNA. Cancer Res. 1979 Jul;39(7 Pt 1):2556–2559. [PubMed] [Google Scholar]
- Phillips D. H., Grover P. L., Sims P. The covalent binding of polycyclic hydrocarbons to DNA in the skin of mice of different strains. Int J Cancer. 1978 Oct 15;22(4):487–494. doi: 10.1002/ijc.2910220419. [DOI] [PubMed] [Google Scholar]
- Rinsky R. A., Smith A. B., Hornung R., Filloon T. G., Young R. J., Okun A. H., Landrigan P. J. Benzene and leukemia. An epidemiologic risk assessment. N Engl J Med. 1987 Apr 23;316(17):1044–1050. doi: 10.1056/NEJM198704233161702. [DOI] [PubMed] [Google Scholar]
- Snyder R., Jowa L., Witz G., Kalf G., Rushmore T. Formation of reactive metabolites from benzene. Arch Toxicol. 1987;60(1-3):61–64. doi: 10.1007/BF00296948. [DOI] [PubMed] [Google Scholar]
- Stumpf R., Margison G. P., Montesano R., Pegg A. E. Formation and loss of alkylated purines from DNA of hamster liver after administration of dimethylnitrosamine. Cancer Res. 1979 Jan;39(1):50–54. [PubMed] [Google Scholar]
- Swenson D. H., Lawley P. D. Alkylation of deoxyribonucleic acid by carcinogens dimethyl sulphate, ethyl methanesulphonate, N-ethyl-N-nitrosourea and N-methyl-N-nitrosourea. Relative reactivity of the phosphodiester site thymidylyl(3'-5')thymidine. Biochem J. 1978 Jun 1;171(3):575–587. doi: 10.1042/bj1710575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace L. A., Pellizzari E. D. Personal air exposures and breath concentrations of benzene and other volatile hydrocarbons for smokers and nonsmokers. Toxicol Lett. 1987 Jan;35(1):113–116. doi: 10.1016/0378-4274(87)90094-4. [DOI] [PubMed] [Google Scholar]
- Wund-Bisseret F., Barraud-Hadidane B., Dirheimer G. In vivo covalent binding of aminofluorene derivatives to mitochondrial liver DNA of rats pretreated with phenobarbital. Toxicol Lett. 1986 Sep;32(3):227–233. doi: 10.1016/0378-4274(86)90112-8. [DOI] [PubMed] [Google Scholar]


