Abstract
Molecular/theoretical modeling studies have revealed that thyroid hormones and toxic chlorinated aromatic hydrocarbons of environmental significance (for which dioxin or TCDD is the prototype) have similar structural properties that could be important in molecular recognition in biochemical systems. These molecular properties include a somewhat rigid, sterically accessible and polarizable aromatic ring and size-limited, hydrophobic lateral substituents, usually contained in opposite adjoining rings of a diphenyl compound. These molecular properties define the primary binding groups thought to be important in molecular recognition of both types of structures in biochemical systems. Similar molecular reactivities are supported by the demonstration of effective specific binding of thyroid hormones and chlorinated aromatic hydrocarbons with four different proteins, enzymes, or receptor preparations that are known or suspected to be involved in the expression of thyroid hormone activity. These binding interactions represent both aromatic-aromatic (stacking) and molecular cleft-type recognition processes. A multiple protein or multifunctional receptor-ligand binding mechanism model is proposed as a way of visualizing the details and possible role of both the stacking and cleft type molecular recognition factors in the expression of biological activity. The model suggests a means by which hormone-responsive effector-linked sites (possible protein-protein-DNA complexes) can maintain highly structurally specific control of hormone action. Finally, the model also provides a theoretical basis for the design and conduct of further biological experimentation on the molecular mechanism(s) of action of toxic chlorinated aromatic hydrocarbons and thyroid hormones.
Full text
PDF




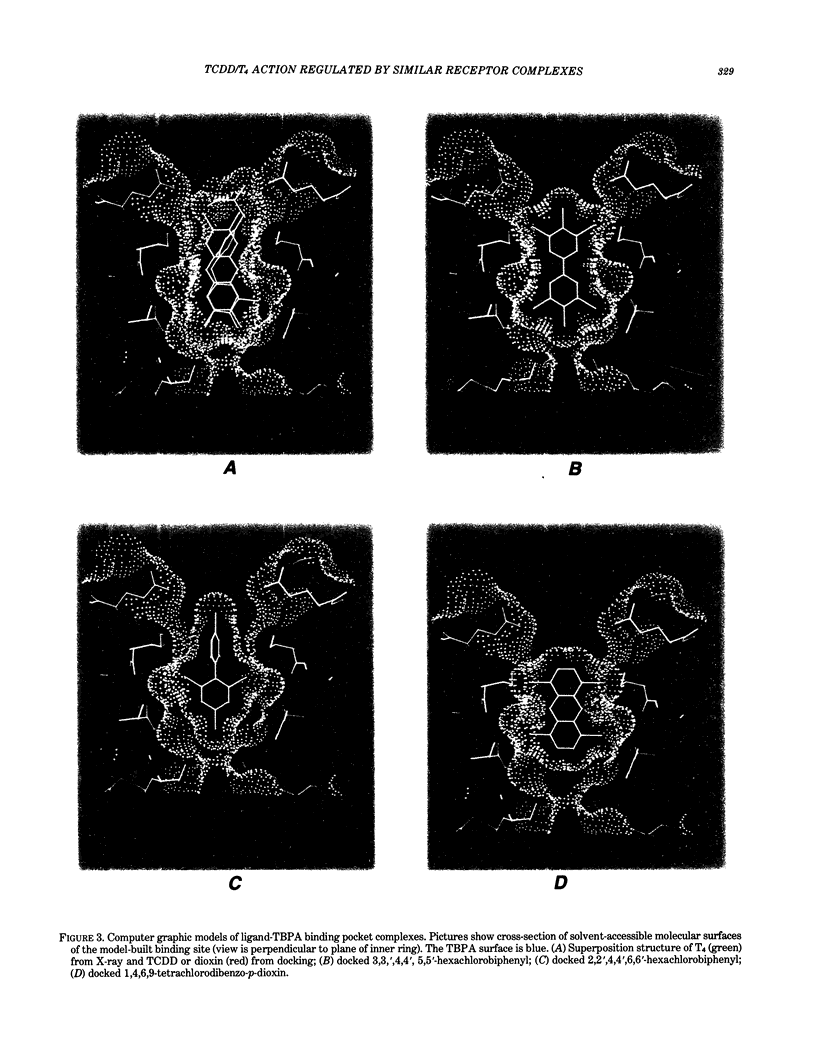

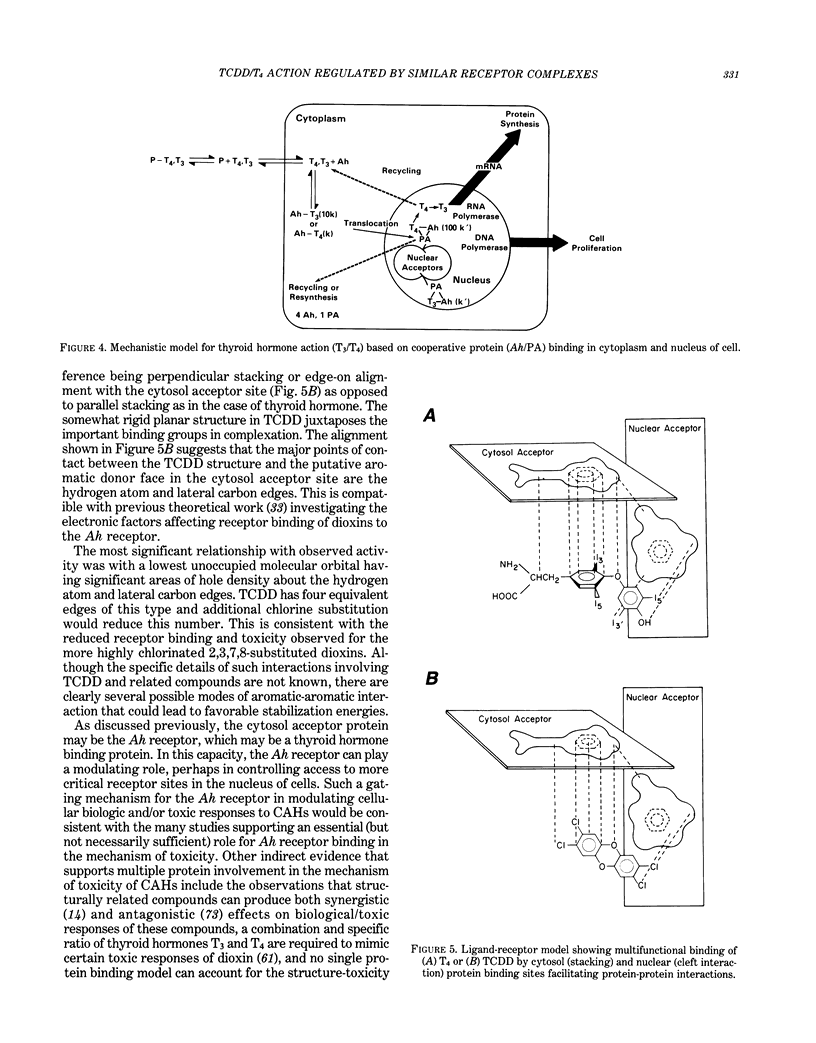
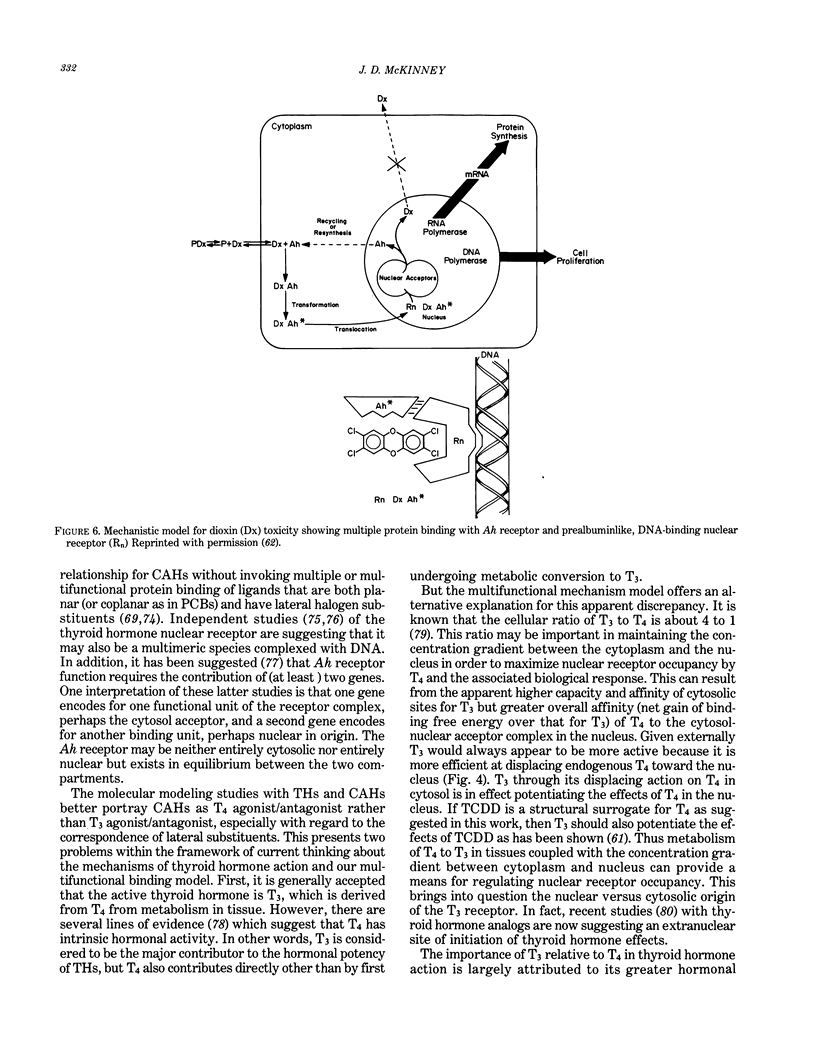

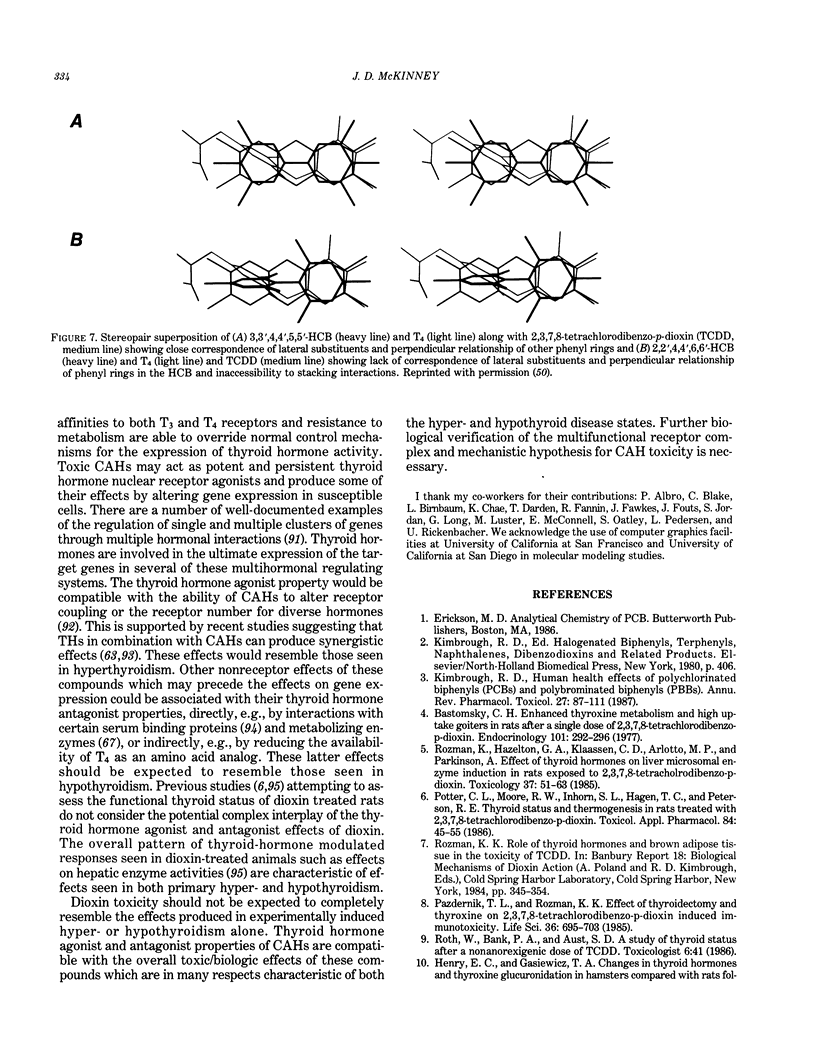


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdukarimov A. Regulation of genetic activity by thyroid hormones. Int Rev Cytol Suppl. 1983;15:17–48. doi: 10.1016/b978-0-12-364376-6.50008-9. [DOI] [PubMed] [Google Scholar]
- Bastomsky C. H. Enhanced thyroxine metabolism and high uptake goiters in rats after a single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Endocrinology. 1977 Jul;101(1):292–296. doi: 10.1210/endo-101-1-292. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Oatley S. J. Protein-DNA and protein-hormone interactions in prealbumin: a model of the thyroid hormone nuclear receptor? Nature. 1977 Jul 14;268(5616):115–120. doi: 10.1038/268115a0. [DOI] [PubMed] [Google Scholar]
- Bolger M. B., Jorgensen E. C. Molecular interactions between thyroid hormone analogs and the rat liver nuclear receptor. Partitioning of equilibrium binding free energy changes into substituent group interactions. J Biol Chem. 1980 Nov 10;255(21):10271–10278. [PubMed] [Google Scholar]
- Bombick D. W., Jankun J., Tullis K., Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes increases in expression of c-erb-A and levels of protein-tyrosine kinases in selected tissues of responsive mouse strains. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4128–4132. doi: 10.1073/pnas.85.12.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C., Guernsey D. L., Ong A., Edelman I. S. Critical role played by thyroid hormone in induction of neoplastic transformation by chemical carcinogens in tissue culture. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5749–5752. doi: 10.1073/pnas.80.18.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayer G. D., McPherson A. Mechanism of DNA binding to the gene 5 protein of bacteriophage fd. Biochemistry. 1984 Jan 17;23(2):340–349. doi: 10.1021/bi00297a025. [DOI] [PubMed] [Google Scholar]
- Brouwer A., van den Berg K. J. Binding of a metabolite of 3,4,3',4'-tetrachlorobiphenyl to transthyretin reduces serum vitamin A transport by inhibiting the formation of the protein complex carrying both retinol and thyroxin. Toxicol Appl Pharmacol. 1986 Sep 30;85(3):301–312. doi: 10.1016/0041-008x(86)90337-6. [DOI] [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Aromatic-aromatic interaction: a mechanism of protein structure stabilization. Science. 1985 Jul 5;229(4708):23–28. doi: 10.1126/science.3892686. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J. M. Tissue distribution of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat. Cancer Res. 1979 Aug;39(8):3172–3176. [PubMed] [Google Scholar]
- Chae K., McKinney J. D. Molecular complexes of thyroid hormone tyrosyl rings with aromatic donors. Possible relationship to receptor protein interactions. J Med Chem. 1988 Feb;31(2):357–362. doi: 10.1021/jm00397a015. [DOI] [PubMed] [Google Scholar]
- Cody V. Thyroid hormone interactions: molecular conformation, protein binding, and hormone action. Endocr Rev. 1980 Spring;1(2):140–166. doi: 10.1210/edrv-1-2-140. [DOI] [PubMed] [Google Scholar]
- Cody V. Thyroid hormones: crystal structure, molecular conformation, binding, and structure-function relationships. Recent Prog Horm Res. 1978;34:437–475. doi: 10.1016/b978-0-12-571134-0.50016-8. [DOI] [PubMed] [Google Scholar]
- DesJarlais R. L., Sheridan R. P., Dixon J. S., Kuntz I. D., Venkataraghavan R. Docking flexible ligands to macromolecular receptors by molecular shape. J Med Chem. 1986 Nov;29(11):2149–2153. doi: 10.1021/jm00161a004. [DOI] [PubMed] [Google Scholar]
- Eberhardt N. L., Ring J. C., Latham K. R., Baxter J. D. Thyroid hormone receptors. Alteration of hormone-binding specificity. J Biol Chem. 1979 Sep 10;254(17):8534–8539. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guernsey D. L., Leuthauser S. W. Correlation of thyroid hormone dose-dependent regulation of K-ras protooncogene expression with oncogene activation by 3-methylcholanthrene: loss of thyroidal regulation in the transformed mouse cell. Cancer Res. 1987 Jun 15;47(12):3052–3056. [PubMed] [Google Scholar]
- Han D. C., Sato K., Fujii Y., Tsushima T., Shizume K. 3,3',5'-Triiodothyronine inhibits iodothyronine-5'-deiodinating activity induced by 3,5,3'-triiodothyronine at equimolar concentrations in cultured fetal mouse liver. Endocrinology. 1986 Sep;119(3):1076–1082. doi: 10.1210/endo-119-3-1076. [DOI] [PubMed] [Google Scholar]
- Henry E. C., Gasiewicz T. A. Changes in thyroid hormones and thyroxine glucuronidation in hamsters compared with rats following treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1987 Jun 30;89(2):165–174. doi: 10.1016/0041-008x(87)90037-8. [DOI] [PubMed] [Google Scholar]
- Hong L. H., McKinney J. D., Luster M. I. Modulation of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated myelotoxicity by thyroid hormones. Biochem Pharmacol. 1987 Apr 15;36(8):1361–1365. doi: 10.1016/0006-2952(87)90095-5. [DOI] [PubMed] [Google Scholar]
- Ishida T., Kamiichi K., Kuwahara A., Doi M., Inoue M. Stacking and hydrogen bonding interactions between phenylalanine and guanine nucleotide: crystal structure of L-phenylalanine-7-methylguanosine-5'-monophosphate complex. Biochem Biophys Res Commun. 1986 Apr 14;136(1):294–299. doi: 10.1016/0006-291x(86)90908-3. [DOI] [PubMed] [Google Scholar]
- JORGENSEN E. C. STEREOCHEMISTRY OF THYROXINE AND ANALOGUES. Mayo Clin Proc. 1964 Aug;39:560–568. [PubMed] [Google Scholar]
- JORGENSEN E. C., WILEY R. A. THYROXINE ANALOGS. VIII. 3-METHYL- AND 3,5-DIMETHYL-DL-THYRONINES AND IODINATED DERIVATIVES. J Med Pharm Chem. 1962 Nov;91:1307–1315. doi: 10.1021/jm01241a022. [DOI] [PubMed] [Google Scholar]
- Jones M. K., Weisenburger W. P., Sipes I. G., Russell D. H. Circadian alterations in prolactin, corticosterone, and thyroid hormone levels and down-regulation of prolactin receptor activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1987 Feb;87(2):337–350. doi: 10.1016/0041-008x(87)90295-x. [DOI] [PubMed] [Google Scholar]
- Jump D. B., Seelig S., Schwartz H. L., Oppenheimer J. H. Association of the thyroid hormone receptor with rat liver chromatin. Biochemistry. 1981 Nov 24;20(24):6781–6789. doi: 10.1021/bi00527a007. [DOI] [PubMed] [Google Scholar]
- Kanda Y., Goodman D. S., Canfield R. E., Morgan F. J. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974 Nov 10;249(21):6796–6805. [PubMed] [Google Scholar]
- Kelling C. K., Menahan L. A., Peterson R. E. Hepatic indices of thyroid status in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biochem Pharmacol. 1987 Jan 15;36(2):283–291. doi: 10.1016/0006-2952(87)90702-7. [DOI] [PubMed] [Google Scholar]
- Keys B., Piskorska-Pliszczynska J., Safe S. Polychlorinated dibenzofurans as 2,3,7,8-TCDD antagonists: in vitro inhibition of monooxygenase enzyme induction. Toxicol Lett. 1986 May;31(2):151–158. doi: 10.1016/0378-4274(86)90009-3. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D. Human health effects of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs). Annu Rev Pharmacol Toxicol. 1987;27:87–111. doi: 10.1146/annurev.pa.27.040187.000511. [DOI] [PubMed] [Google Scholar]
- Koehrle J., Auf'mkolk M., Rokos H., Hesch R. D., Cody V. Rat liver iodothyronine monodeiodinase. Evaluation of the iodothyronine ligand-binding site. J Biol Chem. 1986 Sep 5;261(25):11613–11622. [PubMed] [Google Scholar]
- LOVELOCK J. E. Affinity of organic compounds for free electrons with thermal energy: its possible significance in biology. Nature. 1961 Mar 4;189:729–732. doi: 10.1038/189729a0. [DOI] [PubMed] [Google Scholar]
- Laduron P. M. Criteria for receptor sites in binding studies. Biochem Pharmacol. 1984 Mar 15;33(6):833–839. doi: 10.1016/0006-2952(84)90436-2. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Ioannides C., Parke D. V. Structural requirements for substrates of cytochromes P-450 and P-448. Chem Biol Interact. 1987;64(1-2):39–60. doi: 10.1016/0009-2797(87)90059-7. [DOI] [PubMed] [Google Scholar]
- Manson J. M. Mechanism of nitrofen teratogenesis. Environ Health Perspect. 1986 Dec;70:137–147. doi: 10.1289/ehp.8670137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. S., Tompkins L. S. Effect of o,p'-DDD and similar compounds on thyroxine binding globulin. J Clin Endocrinol Metab. 1968 Mar;28(3):386–392. doi: 10.1210/jcem-28-3-386. [DOI] [PubMed] [Google Scholar]
- Mauchamp J., Shinitzky M. The nature of thyroxine and related compounds as electron donors. Biochemistry. 1969 Apr;8(4):1554–1557. doi: 10.1021/bi00832a034. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Chae K., McConnell E. E., Birnbaum L. S. Structure-induction versus structure-toxicity relationships for polychlorinated biphenyls and related aromatic hydrocarbons. Environ Health Perspect. 1985 May;60:57–68. doi: 10.1289/ehp.856057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney J. D., Chae K., Oatley S. J., Blake C. C. Molecular interactions of toxic chlorinated dibenzo-p-dioxins and dibenzofurans with thyroxine binding prealbumin. J Med Chem. 1985 Mar;28(3):375–381. doi: 10.1021/jm00381a018. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Fawkes J., Jordan S., Chae K., Oatley S., Coleman R. E., Briner W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) as a potent and persistent thyroxine agonist: a mechanistic model for toxicity based on molecular reactivity. Environ Health Perspect. 1985 Sep;61:41–53. doi: 10.1289/ehp.856141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney J. D., Pedersen L. G. Biological activity of polychlorinated biphenyls related to conformational structure. Biochem J. 1986 Dec 1;240(2):621–622. doi: 10.1042/bj2400621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney J. D., Pedersen L. G. Do residue levels of polychlorinated biphenyls (PCBs) in human blood produce mild hypothyroidism? J Theor Biol. 1987 Nov 21;129(2):231–241. doi: 10.1016/s0022-5193(87)80015-2. [DOI] [PubMed] [Google Scholar]
- McKinney J. D. The molecular basis of chemical toxicity. Environ Health Perspect. 1985 Sep;61:5–10. doi: 10.1289/ehp.85615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney J., Fannin R., Jordan S., Chae K., Rickenbacher U., Pedersen L. Polychlorinated biphenyls and related compound interactions with specific binding sites for thyroxine in rat liver nuclear extracts. J Med Chem. 1987 Jan;30(1):79–86. doi: 10.1021/jm00384a014. [DOI] [PubMed] [Google Scholar]
- Mendel C. M., Weisiger R. A., Jones A. L., Cavalieri R. R. Thyroid hormone-binding proteins in plasma facilitate uniform distribution of thyroxine within tissues: a perfused rat liver study. Endocrinology. 1987 May;120(5):1742–1749. doi: 10.1210/endo-120-5-1742. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L. Stereospecific transport of triiodothyronine from plasma to cytosol and from cytosol to nucleus in rat liver, kidney, brain, and heart. J Clin Invest. 1985 Jan;75(1):147–154. doi: 10.1172/JCI111667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Pascual A., Montiel F., Aranda A. Effects of iopanoic acid on thyroid hormone receptor, growth hormone production, and triiodothyronine generation from thyroxine in pituitary GH1 cells. Endocrinology. 1987 Mar;120(3):1089–1096. doi: 10.1210/endo-120-3-1089. [DOI] [PubMed] [Google Scholar]
- Pazdernik T. L., Rozman K. K. Effect of thyroidectomy and thyroxine on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced immunotoxicity. Life Sci. 1985 Feb 18;36(7):695–703. doi: 10.1016/0024-3205(85)90175-4. [DOI] [PubMed] [Google Scholar]
- Pedersen L. G., Darden T. A., Oatley S. J., McKinney J. D. A theoretical study of the binding of polychlorinated biphenyls (PCBs), dibenzodioxins, and dibenzofuran to human plasma prealbumin. J Med Chem. 1986 Dec;29(12):2451–2457. doi: 10.1021/jm00162a006. [DOI] [PubMed] [Google Scholar]
- Perlman A. J., Stanley F., Samuels H. H. Thyroid hormone nuclear receptor. Evidence for multimeric organization in chromatin. J Biol Chem. 1982 Jan 25;257(2):930–938. [PubMed] [Google Scholar]
- Pitot H. C., Goldsworthy T., Campbell H. A., Poland A. Quantitative evaluation of the promotion by 2,3,7,8-tetrachlorodibenzo-p-dioxin of hepatocarcinogenesis from diethylnitrosamine. Cancer Res. 1980 Oct;40(10):3616–3620. [PubMed] [Google Scholar]
- Poellinger L., Gullberg D. Characterization of the hydrophobic properties of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1985 Feb;27(2):271–276. [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Potter C. L., Moore R. W., Inhorn S. L., Hagen T. C., Peterson R. E. Thyroid status and thermogenesis in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1986 Jun 15;84(1):45–55. doi: 10.1016/0041-008x(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Rebek J., Jr Model studies in molecular recognition. Science. 1987 Mar 20;235(4795):1478–1484. doi: 10.1126/science.3823899. [DOI] [PubMed] [Google Scholar]
- Rickenbacher U., McKinney J. D., Oatley S. J., Blake C. C. Structurally specific binding of halogenated biphenyls to thyroxine transport protein. J Med Chem. 1986 May;29(5):641–648. doi: 10.1021/jm00155a010. [DOI] [PubMed] [Google Scholar]
- Rozman K., Hazelton G. A., Klaassen C. D., Arlotto M. P., Parkinson A. Effect of thyroid hormones on liver microsomal enzyme induction in rats exposed to 2,3,7,8,-tetrachlorodibenzo-p-dioxin. Toxicology. 1985 Oct;37(1-2):51–63. doi: 10.1016/0300-483x(85)90112-x. [DOI] [PubMed] [Google Scholar]
- Safe S. H. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol. 1986;26:371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sargent D. F., Schwyzer R. Membrane lipid phase as catalyst for peptide-receptor interactions. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5774–5778. doi: 10.1073/pnas.83.16.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer R. Molecular mechanism of opioid receptor selection. Biochemistry. 1986 Oct 7;25(20):6335–6342. doi: 10.1021/bi00368a075. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K., Bernhard S. A. Metabolite transfer via enzyme-enzyme complexes. Science. 1986 Nov 28;234(4780):1081–1086. doi: 10.1126/science.3775377. [DOI] [PubMed] [Google Scholar]
- Sundelin J., Melhus H., Das S., Eriksson U., Lind P., Trägårdh L., Peterson P. A., Rask L. The primary structure of rabbit and rat prealbumin and a comparison with the tertiary structure of human prealbumin. J Biol Chem. 1985 May 25;260(10):6481–6487. [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Concentration of L-thyroxine and L-triiodothyronine specifically bound to nuclear receptors in rat liver and kidney. Quantitative evidence favoring a major role of T3 in thyroid hormone action. J Clin Invest. 1977 Sep;60(3):555–562. doi: 10.1172/JCI108807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Galeazzi D. R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin receptors in wild type and variant mouse hepatoma cells. Nuclear location and strength of nuclear binding. J Biol Chem. 1984 Jan 25;259(2):980–985. [PubMed] [Google Scholar]
- Whitlock J. P., Jr The regulation of gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Pharmacol Rev. 1987 Jun;39(2):147–161. [PubMed] [Google Scholar]