Abstract
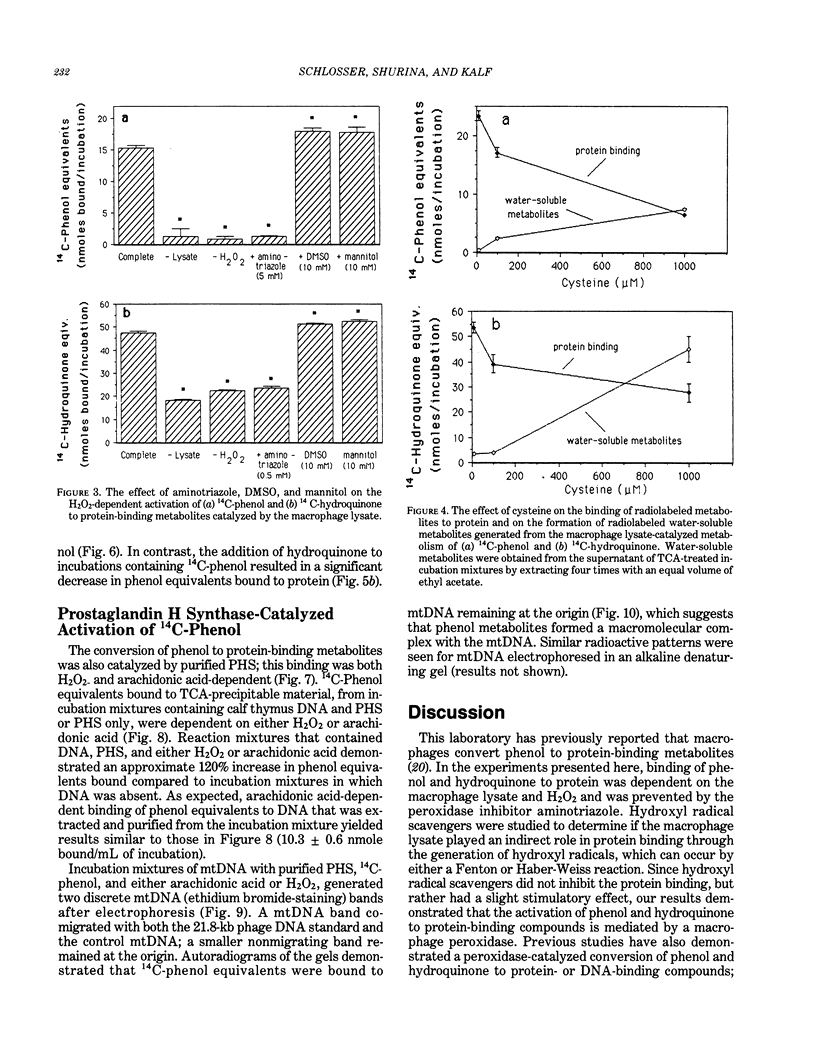
Macrophages, an important cell-type of the bone marrow stroma, are possible targets of benzene toxicity because they contain relatively large amounts of prostaglandin H synthase (PHS), which is capable of metabolizing phenolic compounds to reactive species. PHS also catalyzes the production of prostaglandins, negative regulators of myelopoiesis. Studies indicate that the phenolic metabolites of benzene are oxidized in bone marrow to reactive products via peroxidases. With respect to macrophages, PHS peroxidase is implicated, as in vivo benzene-induced myelotoxicity is prevented by low doses of nonsteroidal anti-inflammatory agents, drugs that inhibit PHS. Incubations of either 14C-phenol or 14C-hydroquinone with a lysate of macrophages collected from mouse peritoneum (greater than 95% macrophages), resulted in an irreversible binding to protein that was dependent upon H2O2, incubation time, and concentration of radiolabel. Production of protein-bound metabolites from phenol or hydroquinone was inhibited by the peroxidase inhibitor aminotriazole. Protein binding from 14C-phenol also was inhibited by 8 microM hydroquinone, whereas binding from 14C-hydroquinone was stimulated by 5 mM phenol. The nucleophile cysteine inhibited protein binding of both phenol and hydroquinone and increased the formation of radiolabeled water-soluble metabolites. Similar to the macrophage lysate, purified PHS also catalyzed the conversion of phenol to metabolites that bound to protein and DNA; this activation was both H2O2- and arachidonic acid-dependent. These results indicate a role for macrophage peroxidase, possibly PHS peroxidase, in the conversion of phenol and hydroquinone to reactive metabolites and suggest that the macrophage should be considered when assessing the hematopoietic toxicity of benzene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Weinstein I. B. Interaction of benzo(a)pyrene and its dihydrodiol-epoxide derivative with nuclear and mitochondrial DNA in C3H10T 1/2 cell cultures. Cancer Res. 1982 Jul;42(7):2764–2769. [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Layman D. L. Regulation of colony-stimulating activity production. Interactions of fibroblasts, mononuclear phagocytes, and lactoferrin. J Clin Invest. 1983 Feb;71(2):340–344. doi: 10.1172/JCI110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodel P. T., Nichols B. A., Bainton D. F. Differences in peroxidase localization of rabbit peritoneal macrophages after surface adherence. Am J Pathol. 1978 Apr;91(1):107–117. [PMC free article] [PubMed] [Google Scholar]
- Boyd J. A., Eling T. E. Prostaglandin H synthase-catalyzed metabolism and DNA binding of 2-naphthylamine. Cancer Res. 1987 Aug 1;47(15):4007–4014. [PubMed] [Google Scholar]
- Brandwein S. R. Regulation of interleukin 1 production by mouse peritoneal macrophages. Effects of arachidonic acid metabolites, cyclic nucleotides, and interferons. J Biol Chem. 1986 Jul 5;261(19):8624–8632. [PubMed] [Google Scholar]
- Breton-Gorius J., Guichard J., Vainchenker W., Vilde J. L. Ultrastructural and cytochemical changes induced by short and prolonged culture of human monocytes. J Reticuloendothel Soc. 1980 Mar;27(3):289–301. [PubMed] [Google Scholar]
- D'Agostino M. A., Lowry K. M., Kalf G. F. DNA biosynthesis in rat liver mitochondria. Inhibition by sulfhydryl compounds and stimulation by cytoplasmic proteins. Arch Biochem Biophys. 1975 Feb;166(2):400–416. doi: 10.1016/0003-9861(75)90403-8. [DOI] [PubMed] [Google Scholar]
- Deimann W., Seitz M., Gemsa D., Fahimi H. D. Endogenous peroxidase in the nuclear envelope and endoplasmic reticulum of human monocytes in vitro: association with arachidonic acid metabolism. Blood. 1984 Aug;64(2):491–498. [PubMed] [Google Scholar]
- Eastmond D. A., Smith M. T., Irons R. D. An interaction of benzene metabolites reproduces the myelotoxicity observed with benzene exposure. Toxicol Appl Pharmacol. 1987 Oct;91(1):85–95. doi: 10.1016/0041-008x(87)90196-7. [DOI] [PubMed] [Google Scholar]
- Eastmond D. A., Smith M. T., Ruzo L. O., Ross D. Metabolic activation of phenol by human myeloperoxidase and horseradish peroxidase. Mol Pharmacol. 1986 Dec;30(6):674–679. [PubMed] [Google Scholar]
- Eling T., Boyd J., Reed G., Mason R., Sivarajah K. Xenobiotic metabolism by prostaglandin endoperoxide synthetase. Drug Metab Rev. 1983;14(5):1023–1053. doi: 10.3109/03602538308991420. [DOI] [PubMed] [Google Scholar]
- Fourney R. M., O'Brien P. J., Davidson W. S. Peroxidase catalyzed aggregation of plasmid pBR322 DNA by benzidine metabolites in vitro. Carcinogenesis. 1986 Sep;7(9):1535–1542. doi: 10.1093/carcin/7.9.1535. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Wierda D. Suppression of bone marrow stromal cell function by benzene and hydroquinone is ameliorated by indomethacin. Toxicol Appl Pharmacol. 1987 Jul;89(3):378–390. doi: 10.1016/0041-008x(87)90157-8. [DOI] [PubMed] [Google Scholar]
- Gentile P. S., Pelus L. M. In vivo modulation of myelopoiesis by prostaglandin E2. II. Inhibition of granulocyte-monocyte progenitor cell (CFU-GM) cell-cycle rate. Exp Hematol. 1987 Feb;15(2):119–126. [PubMed] [Google Scholar]
- Goerig M., Habenicht A. J., Heitz R., Zeh W., Katus H., Kommerell B., Ziegler R., Glomset J. A. sn-1,2-Diacylglycerols and phorbol diesters stimulate thromboxane synthesis by de novo synthesis of prostaglandin H synthase in human promyelocytic leukemia cells. J Clin Invest. 1987 Mar;79(3):903–911. doi: 10.1172/JCI112900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons R. D. Quinones as toxic metabolites of benzene. J Toxicol Environ Health. 1985;16(5):673–678. doi: 10.1080/15287398509530777. [DOI] [PubMed] [Google Scholar]
- Kalf G. F., Schlosser M. J., Renz J. F., Pirozzi S. J. Prevention of benzene-induced myelotoxicity by nonsteroidal anti-inflammatory drugs. Environ Health Perspect. 1989 Jul;82:57–64. doi: 10.1289/ehp.898257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B., Felix C. C., Sealy R. C. Semiquinone anion radicals of catechol(amine)s, catechol estrogens, and their metal ion complexes. Environ Health Perspect. 1985 Dec;64:185–198. doi: 10.1289/ehp.8564185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Ranyard J., Pertcheck M. Myeloperoxidase: its structure and expression during myeloid differentiation. Blood. 1985 Feb;65(2):484–491. [PubMed] [Google Scholar]
- Lee M., Segal G. M., Bagby G. C. Interleukin-1 induces human bone marrow-derived fibroblasts to produce multilineage hematopoietic growth factors. Exp Hematol. 1987 Oct;15(9):983–988. [PubMed] [Google Scholar]
- Lewis J. G., Odom B., Adams D. O. Toxic effects of benzene and benzene metabolites on mononuclear phagocytes. Toxicol Appl Pharmacol. 1988 Feb;92(2):246–254. doi: 10.1016/0041-008x(88)90384-5. [DOI] [PubMed] [Google Scholar]
- Lunte S. M., Kissinger P. T. The use of liquid chromatography with dual-electrode electrochemical detection in the investigation of glutathione oxidation during benzene metabolism. J Chromatogr. 1984 Dec 28;317:579–588. doi: 10.1016/s0021-9673(01)91698-x. [DOI] [PubMed] [Google Scholar]
- Markey C. M., Alward A., Weller P. E., Marnett L. J. Quantitative studies of hydroperoxide reduction by prostaglandin H synthase. Reducing substrate specificity and the relationship of peroxidase to cyclooxygenase activities. J Biol Chem. 1987 May 5;262(13):6266–6279. [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Ohki S., Ogino N., Yamamoto S., Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979 Feb 10;254(3):829–836. [PubMed] [Google Scholar]
- Post G., Snyder R., Kalf G. F. Metabolism of benzene and phenol in macrophages in vitro and the inhibition of RNA synthesis by benzene metabolites. Cell Biol Toxicol. 1986 Jun;2(2):231–246. doi: 10.1007/BF00122692. [DOI] [PubMed] [Google Scholar]
- Rickert D. E., Baker T. S., Bus J. S., Barrow C. S., Irons R. D. Benzene disposition in the rat after exposure by inhalation. Toxicol Appl Pharmacol. 1979 Jul;49(3):417–423. doi: 10.1016/0041-008x(79)90441-1. [DOI] [PubMed] [Google Scholar]
- Rollins T. E., Smith W. L. Subcellular localization of prostaglandin-forming cyclooxygenase in Swiss mouse 3T3 fibroblasts by electron microscopic immunocytochemistry. J Biol Chem. 1980 May 25;255(10):4872–4875. [PubMed] [Google Scholar]
- Romeo D., Cramer R., Marzi T., Soranzo M. R., Zabucchi G., Rossi F. Peroxidase activity of alveolar and peritoneal macrophages. J Reticuloendothel Soc. 1973 May;13(5):399–409. [PubMed] [Google Scholar]
- Rosen G. M., Rauckman E. J., Ellington S. P., Dahlin D. C., Christie J. L., Nelson S. D. Reduction and glutathione conjugation reactions of N-acetyl-p-benzoquinone imine and two dimethylated analogues. Mol Pharmacol. 1984 Jan;25(1):151–157. [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Sammett D., Lee E. W., Kocsis J. J., Snyder R. Partial hepatectomy reduces both metabolism and toxicity of benzene. J Toxicol Environ Health. 1979 Sep;5(5):785–792. doi: 10.1080/15287397909529789. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Zrike J. M., Hamill A. L., Kempe J., Cohn Z. A. Regulation of arachidonic acid metabolites in macrophages. J Exp Med. 1980 Aug 1;152(2):324–335. doi: 10.1084/jem.152.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart R. C., Zannoni V. G. DT-diaphorase and peroxidase influence the covalent binding of the metabolites of phenol, the major metabolite of benzene. Mol Pharmacol. 1984 Jul;26(1):105–111. [PubMed] [Google Scholar]
- Subrahmanyam V. V., O'Brien P. J. Phenol oxidation product(s), formed by a peroxidase reaction, that bind to DNA. Xenobiotica. 1985 Oct;15(10):873–885. doi: 10.3109/00498258509045038. [DOI] [PubMed] [Google Scholar]
- Tagliabue A., Mantovani A., Kilgallen M., Herberman R. B., McCoy J. L. Natural cytotoxicity of mouse monocytes and macrophages. J Immunol. 1979 Jun;122(6):2363–2370. [PubMed] [Google Scholar]
- Thomas D. J., Reasor M. J., Wierda D. Macrophage regulation of myelopoiesis is altered by exposure to the benzene metabolite hydroquinone. Toxicol Appl Pharmacol. 1989 Mar 1;97(3):440–453. doi: 10.1016/0041-008x(89)90249-4. [DOI] [PubMed] [Google Scholar]
- Van Tuyle G. C., McPherson M. L. A compact form of rat liver mitochondrial DNA stabilized by bound proteins. J Biol Chem. 1979 Jul 10;254(13):6044–6053. [PubMed] [Google Scholar]