Abstract
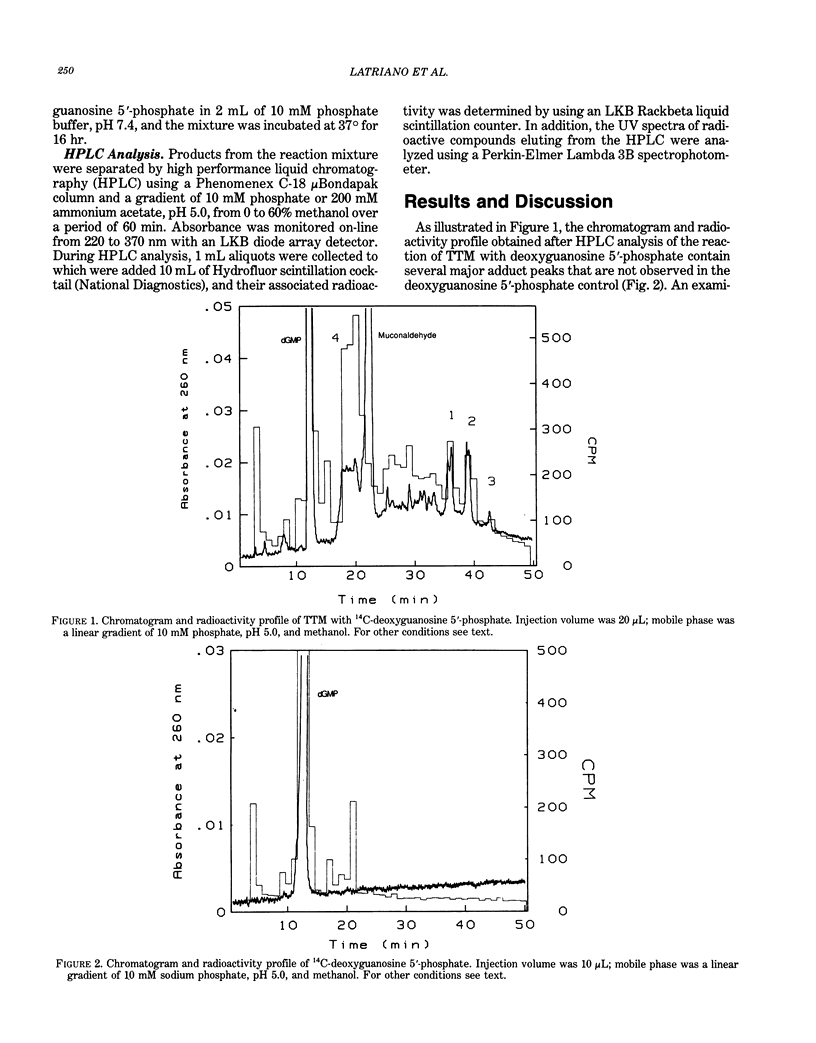
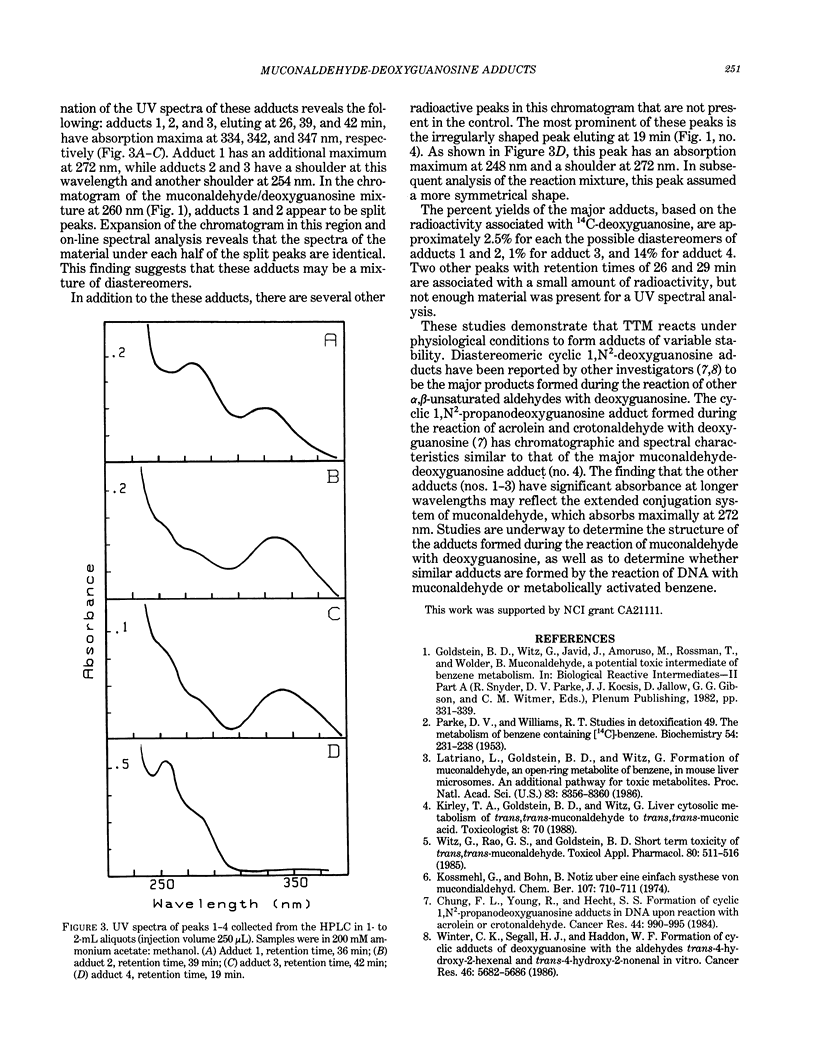
Mice liver microsomes oxidatively open the benzene ring to form trans,trans-muconaldehyde, a hematotoxic unsaturated aldehyde. In the present studies, 4.5 mumole trans,trans-muconaldehyde was reacted with 14C-2'deoxyguanosine 5'-phosphate in phosphate buffer. Products were separated by high performance liquid chromatography (HPLC). Absorbance was monitored using a diode array detector, and aliquots of the HPLC eluant were collected for UV spectrophotometric analysis and scintillation counting. Under these conditions, deoxyguanosine 5'-phosphate eluted at 12.5 min and muconaldehyde at 22.0 min. The HPLC and radioactivity profiles of the muconaldehyde/deoxyguanosine reaction mixture indicated the presence of multiple adducts. Three adducts were detected eluting at 36, 39, and 42 min, which represented approximately 2.5, 2.5, and 1% of the radioactivity, respectively. These adducts had similar UV spectra with absorption maxima between 334 and 347 nm. Another product of the reaction mixture, eluting at 19.0 min and accounting for 10% of the radioactivity, was also observed. This compound had absorption maxima at 348 and 372 nm. These results suggest that trans,trans-muconaldehyde can react with deoxyguanosine monophosphate in vitro under physiological conditions to form stable adducts. Studies are being conducted to determine the structure of these adducts and whether these adducts are formed by the reaction of DNA with muconaldehyde or metabolically activated benzene.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung F. L., Young R., Hecht S. S. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984 Mar;44(3):990–995. [PubMed] [Google Scholar]
- Latriano L., Goldstein B. D., Witz G. Formation of muconaldehyde, an open-ring metabolite of benzene, in mouse liver microsomes: an additional pathway for toxic metabolites. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8356–8360. doi: 10.1073/pnas.83.21.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKE D. V., WILLIAMS R. T. Studies in detoxication. XLIX. The metabolism of benzene containing (14C1) benzene. Biochem J. 1953 May;54(2):231–238. doi: 10.1042/bj0540231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C. K., Segall H. J., Haddon W. F. Formation of cyclic adducts of deoxyguanosine with the aldehydes trans-4-hydroxy-2-hexenal and trans-4-hydroxy-2-nonenal in vitro. Cancer Res. 1986 Nov;46(11):5682–5686. [PubMed] [Google Scholar]
- Witz G., Rao G. S., Goldstein B. D. Short-term toxicity of trans,trans-muconaldehyde. Toxicol Appl Pharmacol. 1985 Sep 30;80(3):511–516. doi: 10.1016/0041-008x(85)90396-5. [DOI] [PubMed] [Google Scholar]


