Abstract
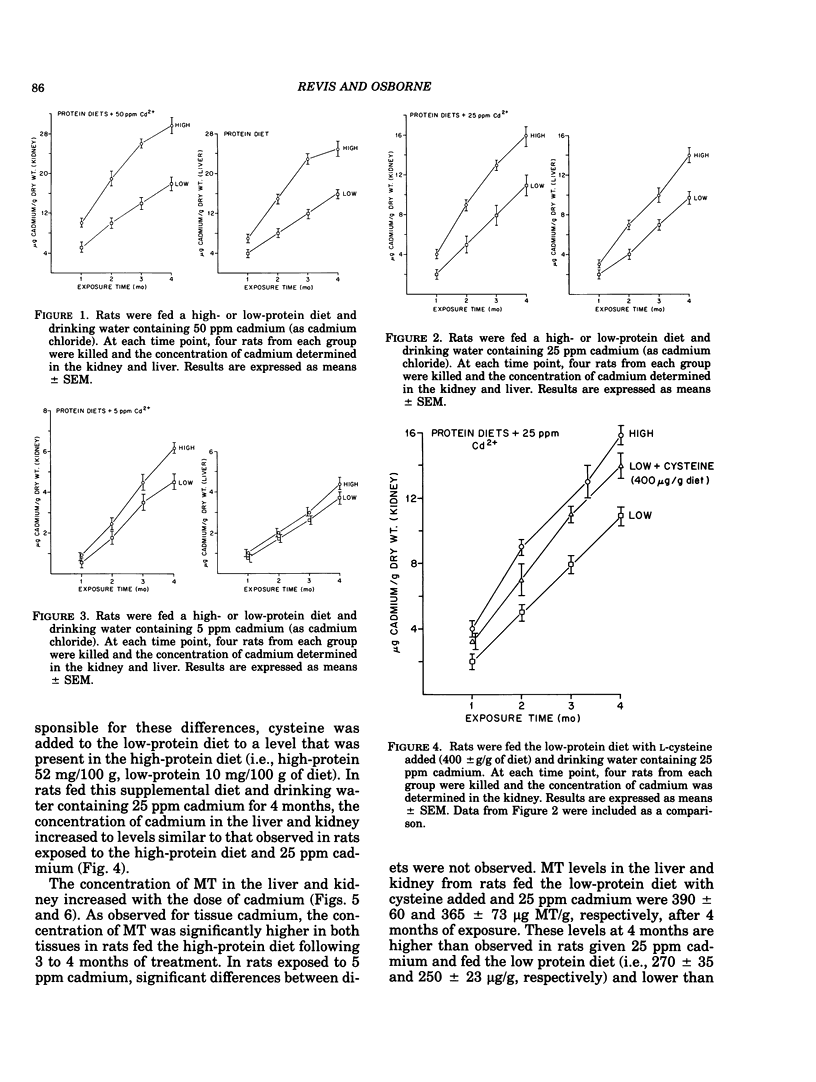
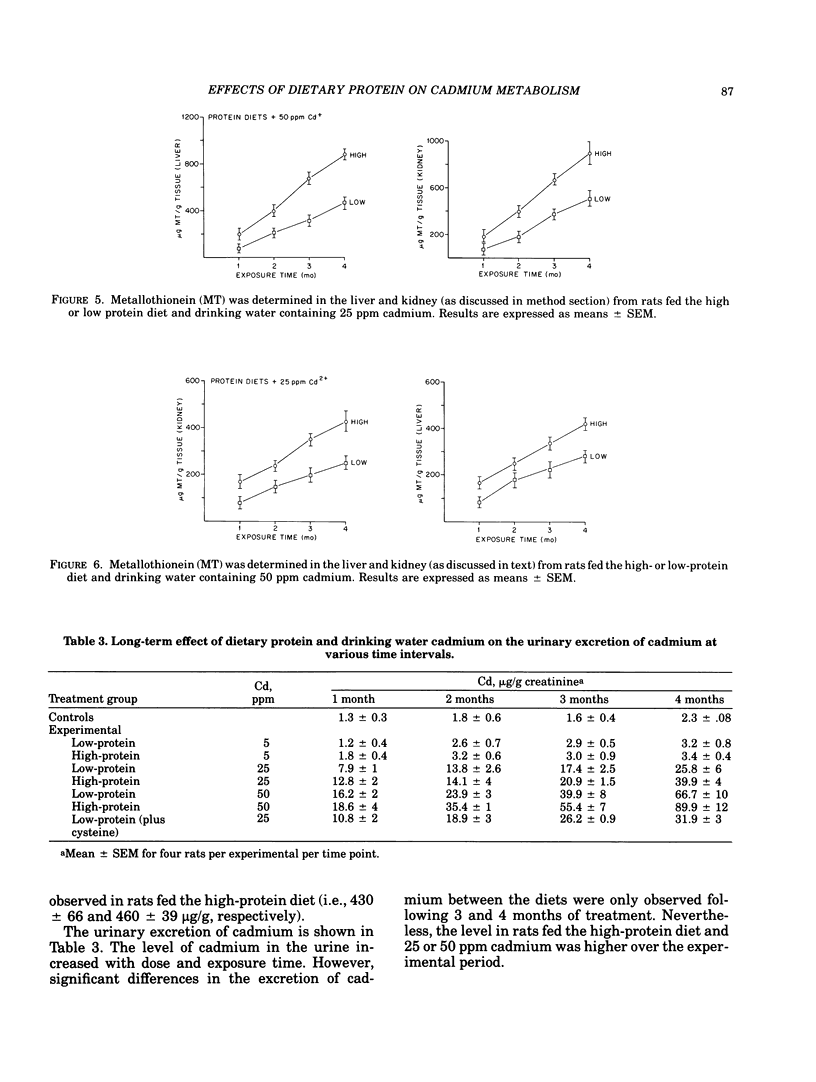
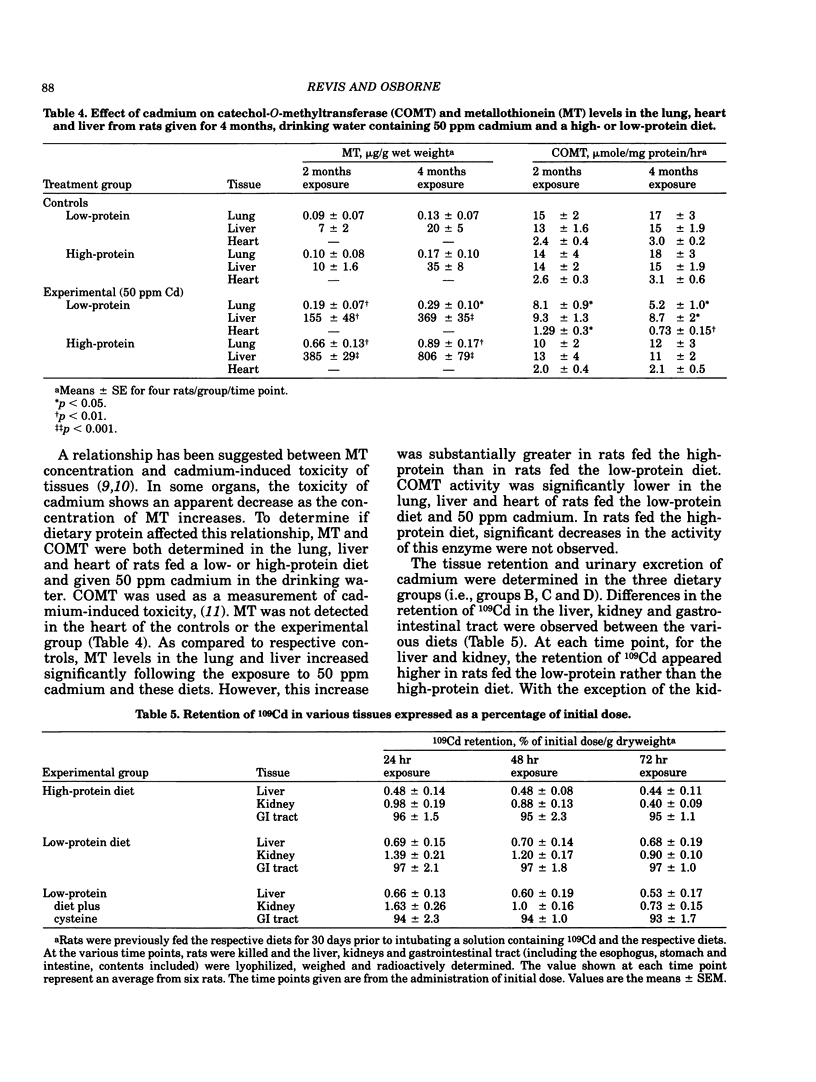
The relationship of dietary protein to cadmium absorption and tissue deposition was studied in male Sprague-Dawley rats exposed to different levels of cadmium in the drinking water. In animals fed a high-protein or low-protein diet and drinking water containing 25 or 50 ppm cadmium, liver and kidney cadmium and metallothionein were both significantly higher in rats fed the high-protein diet for 2 to 4 months. These differences may possibly be explained by the concentration of cysteine observed between these two diets. When cysteine was added to the low-protein diet to the level observed in the high-protein diet and fed to rats receiving 25 ppm cadmium in the drinking water, significant dietary differences in liver and kidney cadmium and metallothionein were not observed. The importance of dietary protein to cadmium-induced toxicity was also assessed in these studies. The activity of catechol-o-methyltransferase was used as a measure of cadmium-induced toxicity. The activity of this enzyme in the lung, liver and heart was significantly lower in rats fed a low-protein diet than those fed the high-protein diet and 50 ppm cadmium. Metallothionein concentration in the lung and liver from low-protein-fed rats was approximately half the level observed in rats fed the high-protein diet, which suggests a relationship between cadmium-induced toxicity and metallothionein concentrations. These results illustrate the importance of considering dietary protein (and possibly cysteine) when studying cadmium metabolism in experimental animals.
Full text
PDF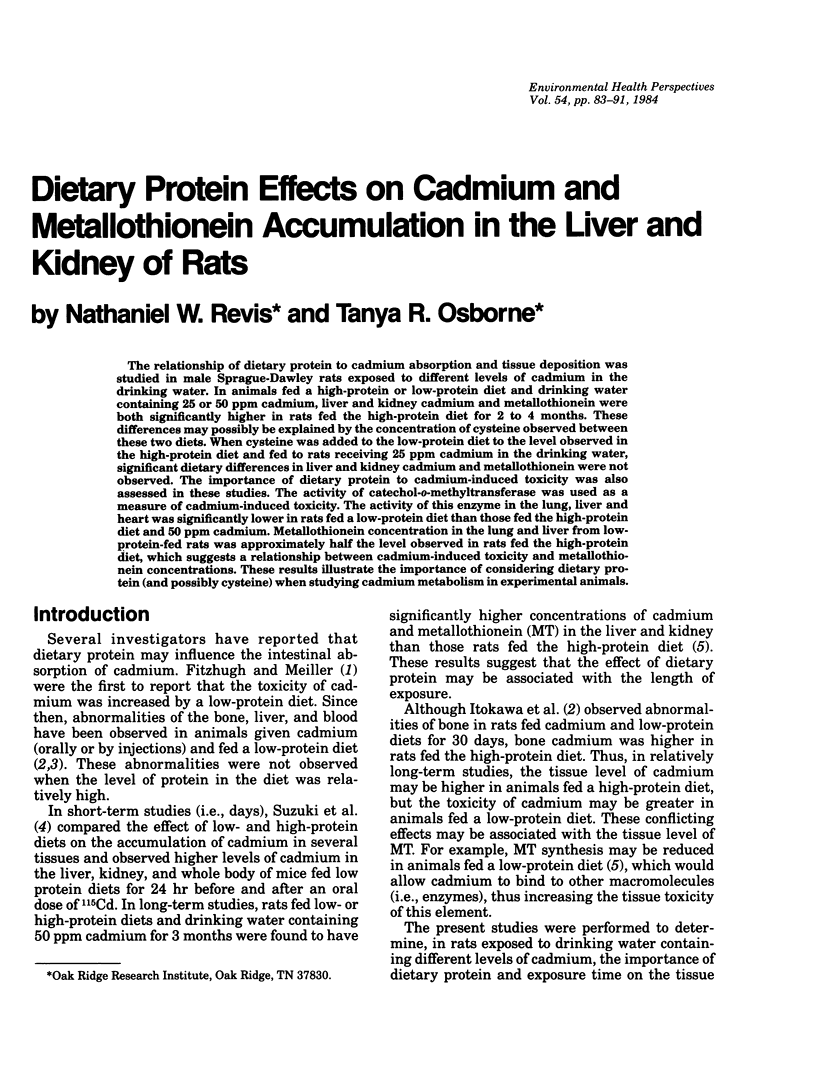





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gontzea I., Popescu F. The effect of body protein supply on resistance to cadmium. Br J Ind Med. 1978 May;35(2):154–160. doi: 10.1136/oem.35.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGSTED D. M., CHANG Y. O. PROTEIN UTILIZATION IN GROWING RATS. I. RELATIVE GROWTH INDEX AS A BIOASSAY PROCEDURE. J Nutr. 1965 Feb;85:159–168. doi: 10.1093/jn/85.2.159. [DOI] [PubMed] [Google Scholar]
- Harper A. E. Effect of variations in protein intake on enzymes of amino acid metabolism. Can J Biochem. 1965 Sep;43(9):1589–1603. doi: 10.1139/o65-176. [DOI] [PubMed] [Google Scholar]
- Itokawa Y., Abe T., Tanaka S. Bone changes in experimental chronic cadmium poisoning: radiological and biological approaches. Arch Environ Health. 1973 May;26(5):241–244. doi: 10.1080/00039896.1973.10666266. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- Kennedy A. The effect of L-cysteine on the toxicity of cadmium. Br J Exp Pathol. 1968 Aug;49(4):360–364. [PMC free article] [PubMed] [Google Scholar]
- Kotsonis F. N., Klaassen C. D. Comparison of methods for estimating hepatic metallothionein in rats. Toxicol Appl Pharmacol. 1977 Dec;42(3):583–588. doi: 10.1016/s0041-008x(77)80043-4. [DOI] [PubMed] [Google Scholar]
- LENZ G. R., MARTELL A. E. METAL CHELATES OF SOME SULFUR-CONTAINING AMINO ACIDS. Biochemistry. 1964 Jun;3:745–750. doi: 10.1021/bi00894a001. [DOI] [PubMed] [Google Scholar]
- McCaman R. E. Microdetermination of catechol-O-methyl transferase in brain. Life Sci. 1965 Dec;4(24):2353–2359. doi: 10.1016/0024-3205(65)90290-0. [DOI] [PubMed] [Google Scholar]
- Murakami M., Webb M. A morphological and biochemical study of the effects of L-cysteine on the renal uptake and nephrotoxicity of cadmium. Br J Exp Pathol. 1981 Apr;62(2):115–130. [PMC free article] [PubMed] [Google Scholar]
- Oh S. H., Whanger P. D. Biological function of metallothionein. VII. Effect of age on its metabolism in rats. Am J Physiol. 1979 Jul;237(1):E18–E22. doi: 10.1152/ajpendo.1979.237.1.E18. [DOI] [PubMed] [Google Scholar]
- PISCATOR M. OM KADMIUM I NORMALA MAENNISKONJURAR SAMT REDOGOERELSE FOER ISOLERING AV METALLOTHIONEIN UR LEVER FRAN KADMIUMEXPONERADE KANINER. Nord Hyg Tidskr. 1964;45:76–82. [PubMed] [Google Scholar]
- Piotrowski J. K., Trojanowska B., Wiśniewska-Knypl J. M., Bolanowska W. Mercury binding in the kidney and liver of rats repeatedly exposed to mercuric chloride: induction of metallothionein by mercury and cadmium. Toxicol Appl Pharmacol. 1974 Jan;27(1):11–19. doi: 10.1016/0041-008x(74)90169-0. [DOI] [PubMed] [Google Scholar]
- Revis N. W. The relationship of dietary protein to metallothionein and cadmium-induced renal damage. Toxicology. 1981;20(4):323–333. doi: 10.1016/0300-483x(81)90039-1. [DOI] [PubMed] [Google Scholar]
- Solomon H. S., Hollenberg N. K. Catecholamine release: mechanism of mercury-induced vascular smooth muscle contraction. Am J Physiol. 1975 Jul;229(1):8–12. doi: 10.1152/ajplegacy.1975.229.1.8. [DOI] [PubMed] [Google Scholar]
- Webb M. Binding of cadmium ions by rat liver and kidney. Biochem Pharmacol. 1972 Oct 15;21(20):2751–2765. doi: 10.1016/0006-2952(72)90023-8. [DOI] [PubMed] [Google Scholar]


