Abstract
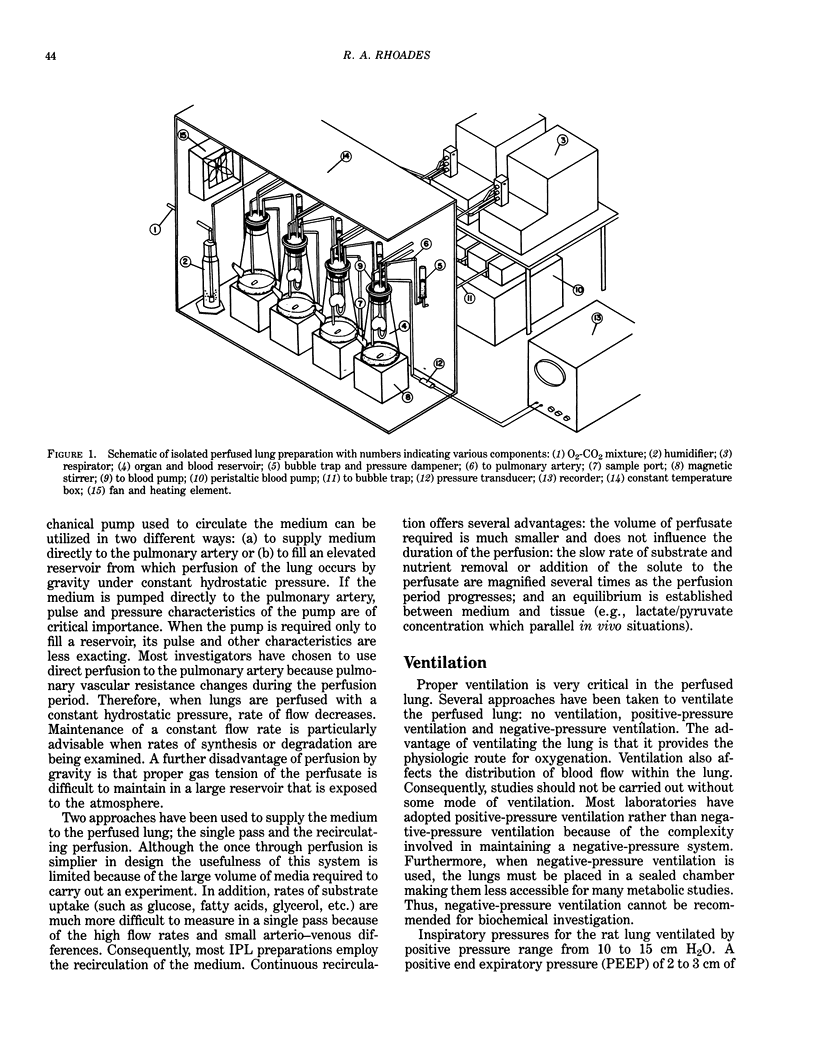
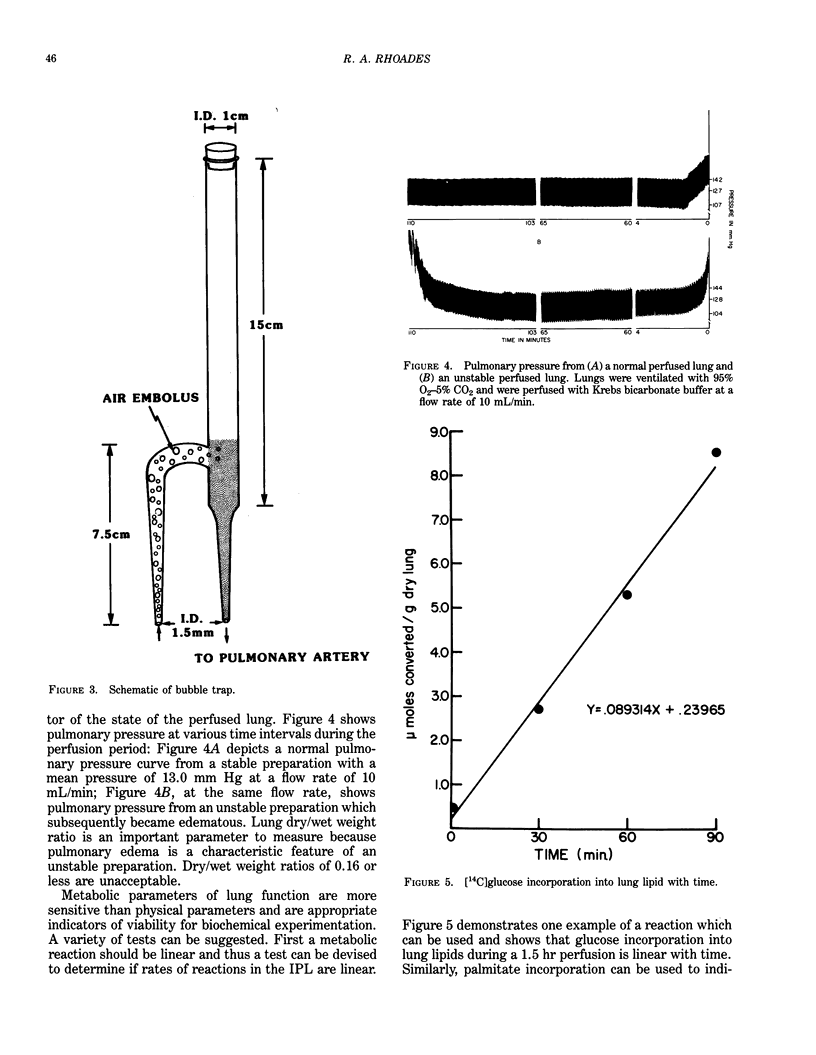
The isolated perfused lung (IPL) preparation is ideally suited to investigate lung dynamics and cellular function, and is easily adapted to investigating biochemical and physiological responses to environmental insults. The IPL offers several advantages which permit one to study endothelial/epithelial interactions that are often disrupted with other model systems (e.g., isolated cells, minces, slices, homogenates, etc.). The IPL developed in our laboratory was devised for the rat lung and allows four lungs to be perfused simultaneously in which control over ventilation, flow, pressure, pH, PO2 and PCO2 can be maintained. Isolated lungs perfused for 1 to 2 hr at a flow rate of 10 mL/min exhibit less that 2% weight gain, maintain normal ATP levels, and exhibit linear substrate uptake. Mechanisms leading to changes in vascular and airway resistance, lipid metabolism, vasoactive hormones, blood gases and changes in vascular permeability mediated by environmental insults can be quantified in the IPL preparation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner E. C., Rhoades R. A. Long-term nitrogen dioxide exposure: effects on lung lipids and mechanical properties. Arch Environ Health. 1973 Mar;26(3):156–160. doi: 10.1080/00039896.1973.10666244. [DOI] [PubMed] [Google Scholar]
- BUELL G. C., TOKIWA Y., MUELLER P. K. POTENTIAL CROSSLINKING AGENTS IN LUNG TISSUE. FORMATION AND ISOLATION AFTER IN VIVO EXPOSURE IN OZONE. Arch Environ Health. 1965 Feb;10:213–219. doi: 10.1080/00039896.1965.10663986. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Glucose metabolism in rat lung during exposure to hyperbaric O2. J Appl Physiol Respir Environ Exerc Physiol. 1979 May;46(5):943–949. doi: 10.1152/jappl.1979.46.5.943. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Metabolic response to carbon monoxide by isolated rat lungs. Am J Physiol. 1976 Mar;230(3):658–663. doi: 10.1152/ajplegacy.1976.230.3.658. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B., Rabinowitz J. L. Effect of hypoxia on incorporation of glucose carbons into lipids by isolated rat lung. Am J Physiol. 1974 Nov;227(5):1103–1108. doi: 10.1152/ajplegacy.1974.227.5.1103. [DOI] [PubMed] [Google Scholar]
- Buechler K. F., Rhoades R. A. Fatty acid synthesis in the perfused rat lung. Biochim Biophys Acta. 1980 Aug 11;619(2):186–195. doi: 10.1016/0005-2760(80)90067-3. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Fisher A. B. Energy status of the rat lung after exposure to elevated PO2. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):56–59. doi: 10.1152/jappl.1978.45.1.56. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Furia L., Chance B. Evaluation of redox state of isolated perfused rat lung. Am J Physiol. 1976 May;230(5):1198–1204. doi: 10.1152/ajplegacy.1976.230.5.1198. [DOI] [PubMed] [Google Scholar]
- Gilder H., McSherry C. K. Mechanisms of oxygen inhibition of pulmonary surfactant synthesis. Surgery. 1974 Jul;76(1):72–79. [PubMed] [Google Scholar]
- Godinez R. I., Longmore W. J. Use of the isolated perfused rat lung in studies on lung lipid metabolism. J Lipid Res. 1973 Mar;14(2):138–144. [PubMed] [Google Scholar]
- Goldstein B. D., Balchum O. J. Effect of ozone on lipid peroxidation in the red blood cell. Proc Soc Exp Biol Med. 1967 Nov;126(2):356–358. doi: 10.3181/00379727-126-32444. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Lodi C., Collinson C., Balchum O. J. Ozone and lipid peroxidation. Arch Environ Health. 1969 Apr;18(4):631–635. doi: 10.1080/00039896.1969.10665464. [DOI] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. S., Baker N. J., Bassett D. J., Fisher A. B. Effect of perfusate glucose concentration on rat lung glycolysis. Am J Physiol. 1979 Mar;236(3):E229–E233. doi: 10.1152/ajpendo.1979.236.3.E229. [DOI] [PubMed] [Google Scholar]
- Levey S., Gast R. Isolated perfused rat lung preparation. J Appl Physiol. 1966 Jan;21(1):313–316. doi: 10.1152/jappl.1966.21.1.313. [DOI] [PubMed] [Google Scholar]
- Lindsey H. E., Wyllie J. H. Release of prostaglandins from embolized lungs. Br J Surg. 1970 Oct;57(10):738–741. doi: 10.1002/bjs.1800571011. [DOI] [PubMed] [Google Scholar]
- Longmore W. J., Mourning J. T. Effect of CO2 concentration on phosphatidylcholine and phosphatidylglycerol metabolism in surfactant and residual lung fractions. J Lipid Res. 1977 May;18(3):309–313. [PubMed] [Google Scholar]
- Longmore W. J., Mourning J. T. Lactate production in isolated perfused rat lung. Am J Physiol. 1976 Aug;231(2):351–354. doi: 10.1152/ajplegacy.1976.231.2.351. [DOI] [PubMed] [Google Scholar]
- Longmore W. J., Niethe C. M., Sprinkle D. J., Godinez R. I. Effect of CO 2 concentration on phospholipid metabolism in the isolated perfused rat lung. J Lipid Res. 1973 Mar;14(2):145–151. [PubMed] [Google Scholar]
- Morgan T. E. Biosynthesis of pulmonary surface-active lipid. Arch Intern Med. 1971 Mar;127(3):401–407. [PubMed] [Google Scholar]
- Morgan T. E., Finley T. N., Huber G. L., Fialkow H. Alterations in pulmonary surface active lipids during exposure to increased oxygen tension. J Clin Invest. 1965 Nov;44(11):1737–1744. doi: 10.1172/JCI105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimark A. Cellular dynamics and lipid metabolism in the lung. Fed Proc. 1973 Sep;32(9):1967–1971. [PubMed] [Google Scholar]
- Newman D., Naimark A. Palmitate-14C uptake by rat lung effect of altered gas tensions. Am J Physiol. 1968 Feb;214(2):305–312. doi: 10.1152/ajplegacy.1968.214.2.305. [DOI] [PubMed] [Google Scholar]
- Rhoades R. A. Net uptake of glucose, glycerol, and fatty acids by the isolated perfused rat lung. Am J Physiol. 1974 Jan;226(1):144–149. doi: 10.1152/ajplegacy.1974.226.1.144. [DOI] [PubMed] [Google Scholar]
- Rhoades R. A. Perfused lung preparation for studying altered gaseous environments. Environ Health Perspect. 1976 Aug;16:73–75. doi: 10.1289/ehp.761673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades R. A., Shaw M. E., Eskew M. L. Influence of altered O2 tension on substrate metabolism in perfused rat lung. Am J Physiol. 1975 Dec;229(6):1476–1479. doi: 10.1152/ajplegacy.1975.229.6.1476. [DOI] [PubMed] [Google Scholar]
- Rhoades R. A., Shaw M. E., Eskew M. L., Wali S. Lactate metabolism in perfused rat lung. Am J Physiol. 1978 Dec;235(6):E619–E623. doi: 10.1152/ajpendo.1978.235.6.E619. [DOI] [PubMed] [Google Scholar]
- Shaw M. E., Rhoades R. A. Substrate metabolism in the perfused lung: response to changes in circulating glucose and palmitate levels. Lipids. 1977 Nov;12(11):930–935. doi: 10.1007/BF02533313. [DOI] [PubMed] [Google Scholar]
- Thomas H. V., Mueller P. K., Lyman R. L. Lipoperoxidation of lung lipids in rats exposed to nitrogen dioxide. Science. 1968 Feb 2;159(3814):532–534. doi: 10.1126/science.159.3814.532. [DOI] [PubMed] [Google Scholar]
- Thomas T., Jr, Rhoades R. A. 14C-1-palmitate incorporation by rat lung: effect of nitrogen dioxide. Proc Soc Exp Biol Med. 1970 Sep;134(4):1181–1183. doi: 10.3181/00379727-134-34969. [DOI] [PubMed] [Google Scholar]
- Välimäki M., Pelliniemi T. T., Niinikoski J. Oxygen-induced changes in pulmonary phospholipids in the rat. J Appl Physiol. 1975 Nov;39(5):780–787. doi: 10.1152/jappl.1975.39.5.780. [DOI] [PubMed] [Google Scholar]
- Watkins C. A., Rannels D. E. In situ perfusion of rat lungs: stability and effects of oxygen tension. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):325–329. doi: 10.1152/jappl.1979.47.2.325. [DOI] [PubMed] [Google Scholar]
- Weber K. C., Visscher M. B. Metabolism of the isolated canine lung. Am J Physiol. 1969 Oct;217(4):1044–1052. doi: 10.1152/ajplegacy.1969.217.4.1044. [DOI] [PubMed] [Google Scholar]


