Abstract
The carcinogenicity database used for this paper originated in the late 1960s by the National Cancer Institute (NCI) and since 1978 has been continued and made more comprehensive by the National Toxicology Program (NTP). The extensive files contain, among other sets of information, detailed pathology data on more than 400 long-term (most often 24-month) chemical carcinogenesis studies, comprising nearly 1600 individual experiments having at least 10 million tissue sections that have been evaluated for toxicity and carcinogenicity.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Chhabra R. S., Huff J. E., Schwetz B. S., Selkirk J. An overview of prechronic and chronic toxicity/carcinogenicity experimental study designs and criteria used by the National Toxicology Program. Environ Health Perspect. 1990 Jun;86:313–321. doi: 10.1289/ehp.9086313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustis S. L. The sequential development of cancer: a morphological perspective. Toxicol Lett. 1989 Dec;49(2-3):267–281. doi: 10.1016/0378-4274(89)90037-4. [DOI] [PubMed] [Google Scholar]
- Haseman J. K. A reexamination of false-positive rates for carcinogenesis studies. Fundam Appl Toxicol. 1983 Jul-Aug;3(4):334–339. doi: 10.1016/s0272-0590(83)80148-1. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Clark A. M. Carcinogenicity results for 114 laboratory animal studies used to assess the predictivity of four in vitro genetic toxicity assays for rodent carcinogenicity. Environ Mol Mutagen. 1990;16 (Suppl 18):15–31. doi: 10.1002/em.2850160503. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J. E., Rao G. N., Arnold J. E., Boorman G. A., McConnell E. E. Neoplasms observed in untreated and corn oil gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst. 1985 Nov;75(5):975–984. doi: 10.1093/jnci/75.5.975. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J. E., Rao G. N., Eustis S. L. Sources of variability in rodent carcinogenicity studies. Fundam Appl Toxicol. 1989 May;12(4):793–804. doi: 10.1093/toxsci/12.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
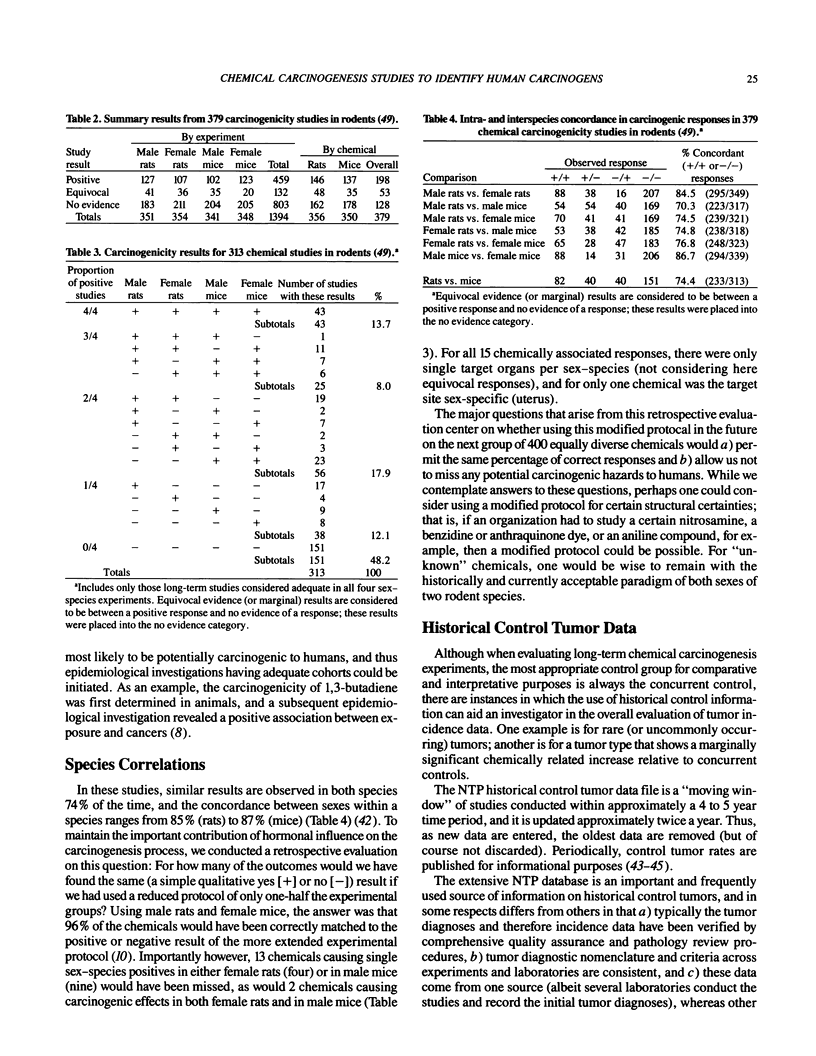
- Haseman J. K., Huff J. E. Species correlation in long-term carcinogenicity studies. Cancer Lett. 1987 Oct 30;37(2):125–132. doi: 10.1016/0304-3835(87)90154-6. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J. E., Zeiger E., McConnell E. E. Comparative results of 327 chemical carcinogenicity studies. Environ Health Perspect. 1987 Oct;74:229–235. doi: 10.1289/ehp.8774229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman J. K., Huff J., Boorman G. A. Use of historical control data in carcinogenicity studies in rodents. Toxicol Pathol. 1984;12(2):126–135. doi: 10.1177/019262338401200203. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Tharrington E. C., Huff J. E., McConnell E. E. Comparison of site-specific and overall tumor incidence analyses for 81 recent National Toxicology Program carcinogenicity studies. Regul Toxicol Pharmacol. 1986 Jun;6(2):155–170. doi: 10.1016/0273-2300(86)90031-0. [DOI] [PubMed] [Google Scholar]
- Haseman J. K. Use of statistical decision rules for evaluating laboratory animal carcinogenicity studies. Fundam Appl Toxicol. 1990 May;14(4):637–648. doi: 10.1016/0272-0590(90)90289-v. [DOI] [PubMed] [Google Scholar]
- Hoel D. G., Haseman J. K., Hogan M. D., Huff J., McConnell E. E. The impact of toxicity on carcinogenicity studies: implications for risk assessment. Carcinogenesis. 1988 Nov;9(11):2045–2052. doi: 10.1093/carcin/9.11.2045. [DOI] [PubMed] [Google Scholar]
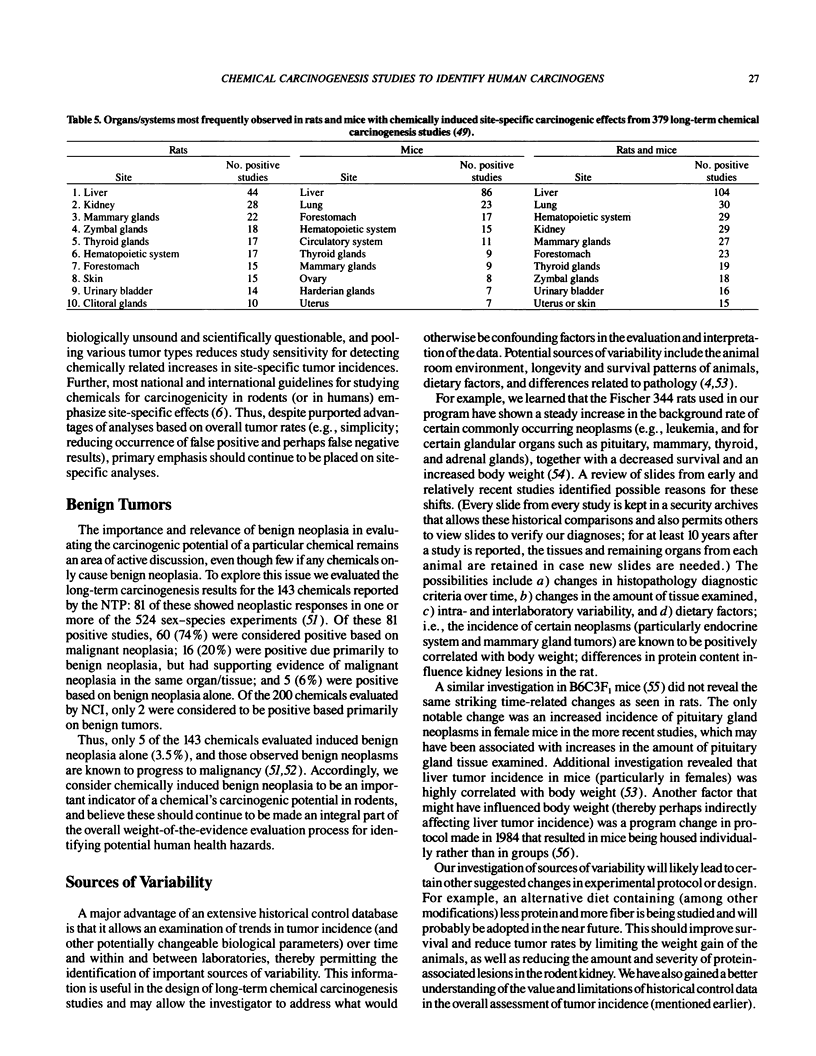
- Huff J. E., Eustis S. L., Haseman J. K. Occurrence and relevance of chemically induced benign neoplasms in long-term carcinogenicity studies. Cancer Metastasis Rev. 1989 Jun;8(1):1–22. doi: 10.1007/BF00047055. [DOI] [PubMed] [Google Scholar]
- Huff J. E., McConnell E. E., Haseman J. K., Boorman G. A., Eustis S. L., Schwetz B. A., Rao G. N., Jameson C. W., Hart L. G., Rall D. P. Carcinogenesis studies: results of 398 experiments on 104 chemicals from the U.S. National Toxicology Program. Ann N Y Acad Sci. 1988;534:1–30. doi: 10.1111/j.1749-6632.1988.tb30085.x. [DOI] [PubMed] [Google Scholar]
- Huff J. E., Moore J. A. Carcinogenesis studies design and experimental data interpretation/evaluation at the National Toxicology Program. Prog Clin Biol Res. 1984;141:43–64. [PubMed] [Google Scholar]
- Huff J., Cirvello J., Haseman J., Bucher J. Chemicals associated with site-specific neoplasia in 1394 long-term carcinogenesis experiments in laboratory rodents. Environ Health Perspect. 1991 Jun;93:247–270. doi: 10.1289/ehp.9193247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J., Haseman J., Rall D. Scientific concepts, value, and significance of chemical carcinogenesis studies. Annu Rev Pharmacol Toxicol. 1991;31:621–652. doi: 10.1146/annurev.pa.31.040191.003201. [DOI] [PubMed] [Google Scholar]
- Maronpot R. R., Haseman J. K., Boorman G. A., Eustis S. E., Rao G. N., Huff J. E. Liver lesions in B6C3F1 mice: the National Toxicology Program, experience and position. Arch Toxicol Suppl. 1987;10:10–26. doi: 10.1007/978-3-642-71617-1_2. [DOI] [PubMed] [Google Scholar]
- Melnick R. L., Jameson C. W., Goehl T. J., Kuhn G. O. Application of microencapsulation for toxicology studies. I. Principles and stabilization of trichloroethylene in gelatin-sorbitol microcapsules. Fundam Appl Toxicol. 1987 May;8(4):425–431. doi: 10.1016/0272-0590(87)90128-x. [DOI] [PubMed] [Google Scholar]
- Muir C. S. Epidemiology, basic science, and the prevention of cancer: implications for the future. Cancer Res. 1990 Oct 15;50(20):6441–6448. [PubMed] [Google Scholar]
- Rao G. N., Haseman J. K., Grumbein S., Crawford D. D., Eustis S. L. Growth, body weight, survival, and tumor trends in (C57BL/6 X C3H/HeN) F1 (B6C3F1) mice during a nine-year period. Toxicol Pathol. 1990;18(1 Pt 1):71–77. doi: 10.1177/019262339001800110. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Haseman J. K., Grumbein S., Crawford D. D., Eustis S. L. Growth, body weight, survival, and tumor trends in F344/N rats during an eleven-year period. Toxicol Pathol. 1990;18(1 Pt 1):61–70. doi: 10.1177/019262339001800109. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Huff J. Refinement of long-term toxicity and carcinogenesis studies. Fundam Appl Toxicol. 1990 Jul;15(1):33–43. doi: 10.1016/0272-0590(90)90160-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Elwell M. R., Spalding J. W., Griesemer R. A. Evidence that toxic injury is not always associated with induction of chemical carcinogenesis. Mol Carcinog. 1991;4(6):420–440. doi: 10.1002/mc.2940040604. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Margolin B. H., Shelby M. D., Zeiger E., Haseman J. K., Spalding J., Caspary W., Resnick M., Stasiewicz S., Anderson B. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–941. doi: 10.1126/science.3554512. [DOI] [PubMed] [Google Scholar]
- Tennant R. W. The genetic toxicity database of the National Toxicology Program: evaluation of the relationships between genetic toxicity and carcinogenicity. Environ Health Perspect. 1991 Dec;96:47–51. doi: 10.1289/ehp.919647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatis L., Aitio A., Wilbourn J., Shuker L. Human carcinogens so far identified. Jpn J Cancer Res. 1989 Sep;80(9):795–807. doi: 10.1111/j.1349-7006.1989.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Weinstein I. B. Mitogenesis is only one factor in carcinogenesis. Science. 1991 Jan 25;251(4992):387–388. doi: 10.1126/science.1989073. [DOI] [PubMed] [Google Scholar]
- You M., Candrian U., Maronpot R. R., Stoner G. D., Anderson M. W. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci U S A. 1989 May;86(9):3070–3074. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E., Haseman J. K., Shelby M. D., Margolin B. H., Tennant R. W. Evaluation of four in vitro genetic toxicity tests for predicting rodent carcinogenicity: confirmation of earlier results with 41 additional chemicals. Environ Mol Mutagen. 1990;16 (Suppl 18):1–14. doi: 10.1002/em.2850160502. [DOI] [PubMed] [Google Scholar]



