Abstract
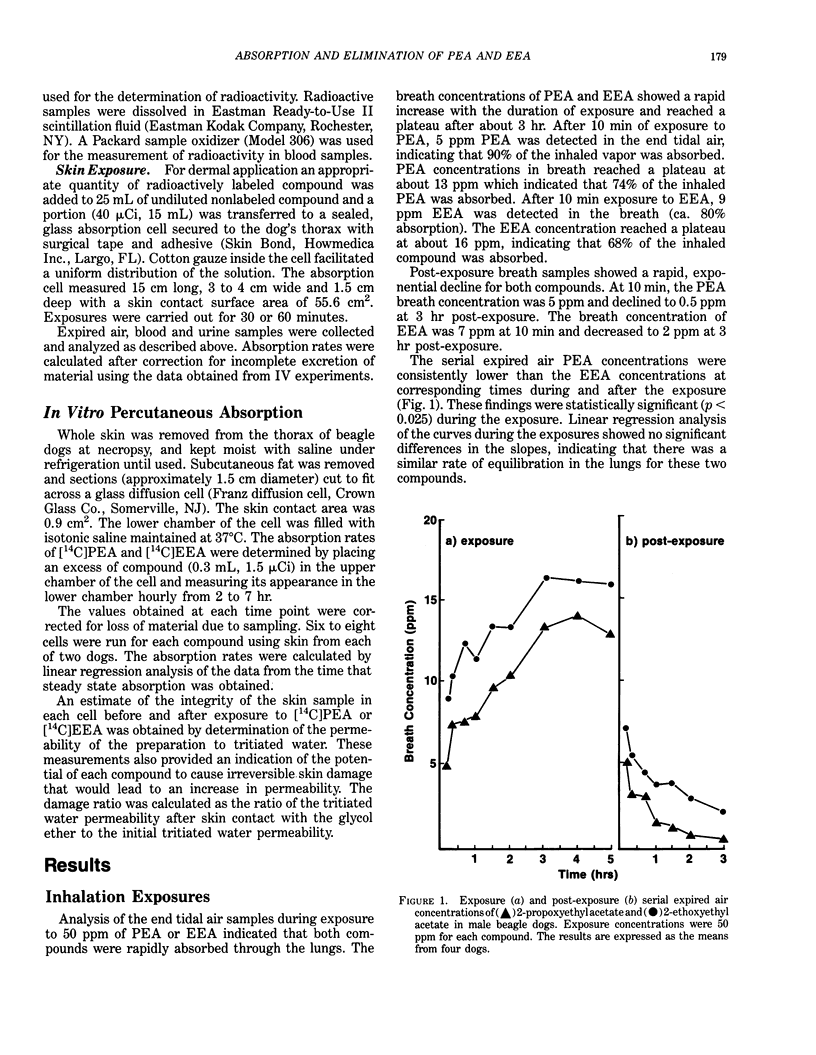
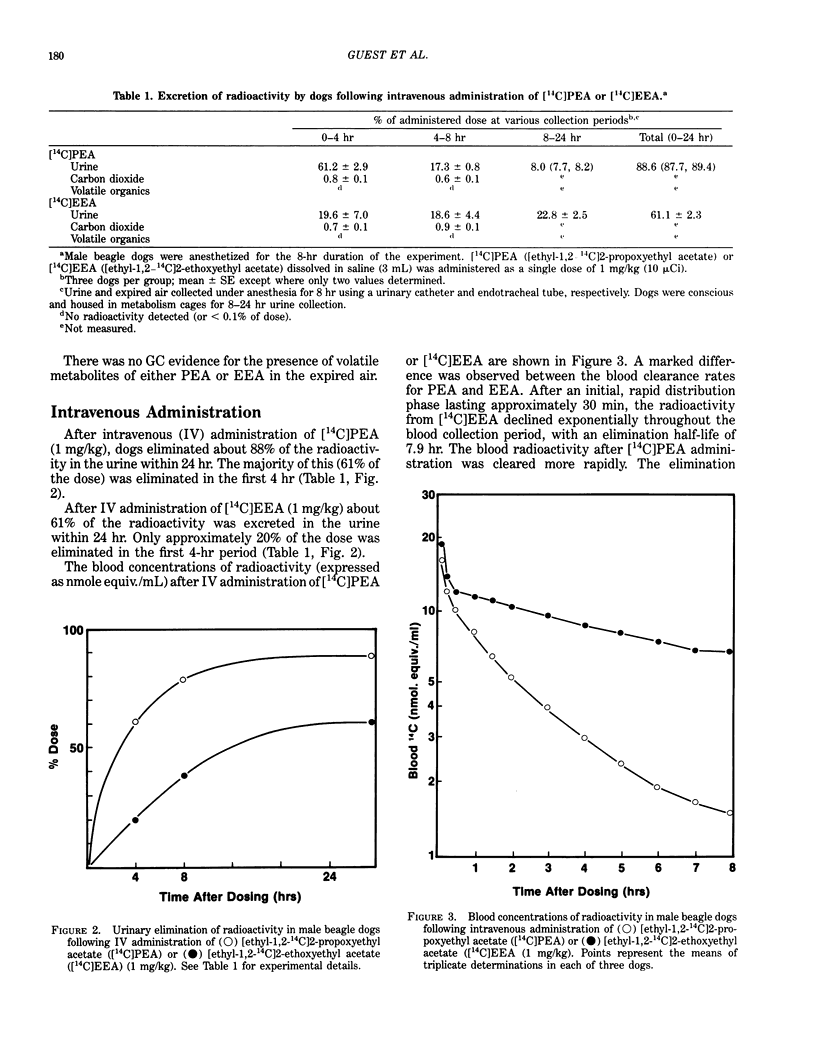
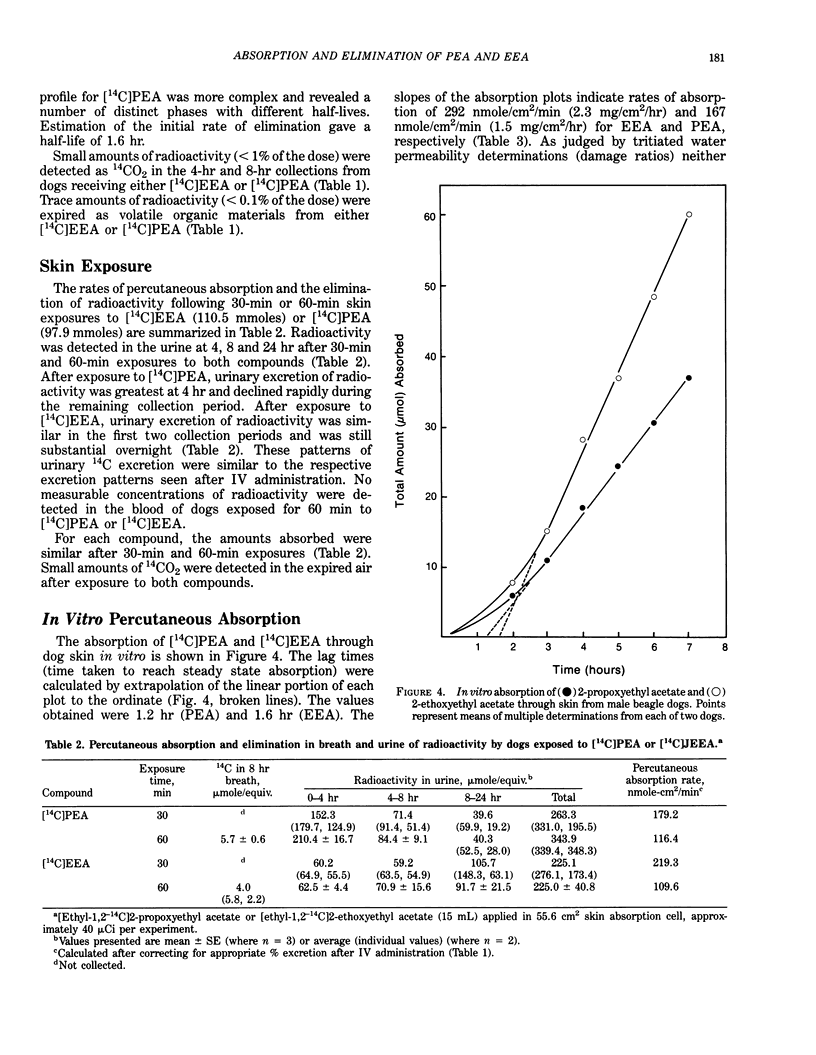
A comparison was made of the absorption and elimination rates of 2-propoxyethyl acetate (PEA) and 2-ethoxyethyl acetate (EEA) following inhalation, dermal application or IV administration. Male beagle dogs were exposed to 50 ppm PEA or EEA for 5 hr, and breath samples were collected during the exposure and a 3-hr recovery period. Both compounds were rapidly absorbed through the lungs. After 10 min of exposure, the concentrations of the parent compounds in the expired breath were 5 to 10 ppm (80-90% absorption) and reached plateau values at about 3 hr of 13 ppm for PEA (74% absorption) and 16 ppm for EEA (68% absorption). Post-exposure breath samples declined exponentially to 0.5 ppm and 2 ppm after 3 hr for PEA and EEA, respectively. Expired concentrations of PEA were slightly, but significantly (p less than 0.025), lower than those of EEA at corresponding times during the exposure. After IV dosing with 1 mg/kg [ethyl-1,2-14C]PEA, the urine contained 61% and 88% of the dose in 4 and 24 hr, respectively. [14C]EEA was eliminated more slowly, with 20% and 61% of the dose appearing in the urine in 4 and 24 hr, respectively. Blood elimination half-lives were 1.6 hr for [14C]PEA and 7.9 hr for [14C]EEA. Only trace amounts of 14CO2 (less than 1%) or volatile materials (less than 0.1%) were detected in the expired air with either compound. For studies of percutaneous absorption, [14C]PEA or [14C]EEA was added to undiluted compound and applied in a glass cell to a shaved area on a dog's thorax for 30 or 60 min.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheever K. L., Plotnick H. B., Richards D. E., Weigel W. W. Metabolism and excretion of 2-ethoxyethanol in the adult male rat. Environ Health Perspect. 1984 Aug;57:241–248. doi: 10.1289/ehp.8457241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVincenzo G. D., Hamilton M. L. Fate of n-butanol in rats after oral administration and its uptake by dogs after inhalation or skin application. Toxicol Appl Pharmacol. 1979 Apr;48(2):317–325. doi: 10.1016/0041-008x(79)90038-3. [DOI] [PubMed] [Google Scholar]
- DiVincenzo G. D., Hamilton M. L., Kaplan C. J., Krasavage W. J., O'Donoghue J. L. Studies on the respiratory uptake and excretion and the skin absorption of methyl n-butyl ketone in humans and dogs. Toxicol Appl Pharmacol. 1978 Jun;44(3):593–604. doi: 10.1016/0041-008x(78)90267-3. [DOI] [PubMed] [Google Scholar]
- Hardin B. D., Bond G. P., Sikov M. R., Andrew F. D., Beliles R. P., Niemeier R. W. Testing of selected workplace chemicals for teratogenic potential. Scand J Work Environ Health. 1981;7 (Suppl 4):66–75. [PubMed] [Google Scholar]
- Jönsson A. K., Pedersen J., Steen G. Ethoxyacetic acid and N-ethoxyacetylglycine: metabolites of ethoxyethanol (ethylcellosolve) in rats. Acta Pharmacol Toxicol (Copenh) 1982 May;50(5):358–362. doi: 10.1111/j.1600-0773.1982.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Jönsson A. K., Steen G. n-Butoxyacetic acid, a urinary metabolite from inhaled n-butoxyethanol (Butylcellosolve). Acta Pharmacol Toxicol (Copenh) 1978 May;42(5):354–356. doi: 10.1111/j.1600-0773.1978.tb02216.x. [DOI] [PubMed] [Google Scholar]
- Kamerling J. P., Duran M., Bruinvis L., Ketting D., Wadman S. K., de Groot C. J., Hommes F. A. (2-Ethoxyethoxy)acetic acid: an unusual compound found in the gas chromatographic analysis of urinary organic acids. Clin Chim Acta. 1977 Jun 15;77(3):397–405. doi: 10.1016/0009-8981(77)90246-7. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Ayres J. A., Calhoun L. L., Young J. T., McKenna M. J. Comparative short-term inhalation toxicity of ethylene glycol monomethyl ether and propylene glycol monomethyl ether in rats and mouse. Toxicol Appl Pharmacol. 1981 Dec;61(3):368–377. doi: 10.1016/0041-008x(81)90358-6. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Hermann E. A., Langvardt P. W., McKenna M. J., Schwetz B. A. Comparative metabolism and disposition of ethylene glycol monomethyl ether and propylene glycol monomethyl ether in male rats. Toxicol Appl Pharmacol. 1983 Feb;67(2):229–237. doi: 10.1016/0041-008x(83)90229-6. [DOI] [PubMed] [Google Scholar]
- Nagano K., Nakayama E., Oobayashi H., Yamada T., Adachi H., Nishizawa T., Ozawa H., Nakaichi M., Okuda H., Minami K. Embryotoxic effects of ethylene glycol monomethyl ether in mice. Toxicology. 1981;20(4):335–343. doi: 10.1016/0300-483x(81)90040-8. [DOI] [PubMed] [Google Scholar]


