Abstract
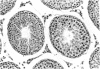
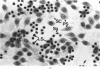
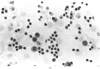
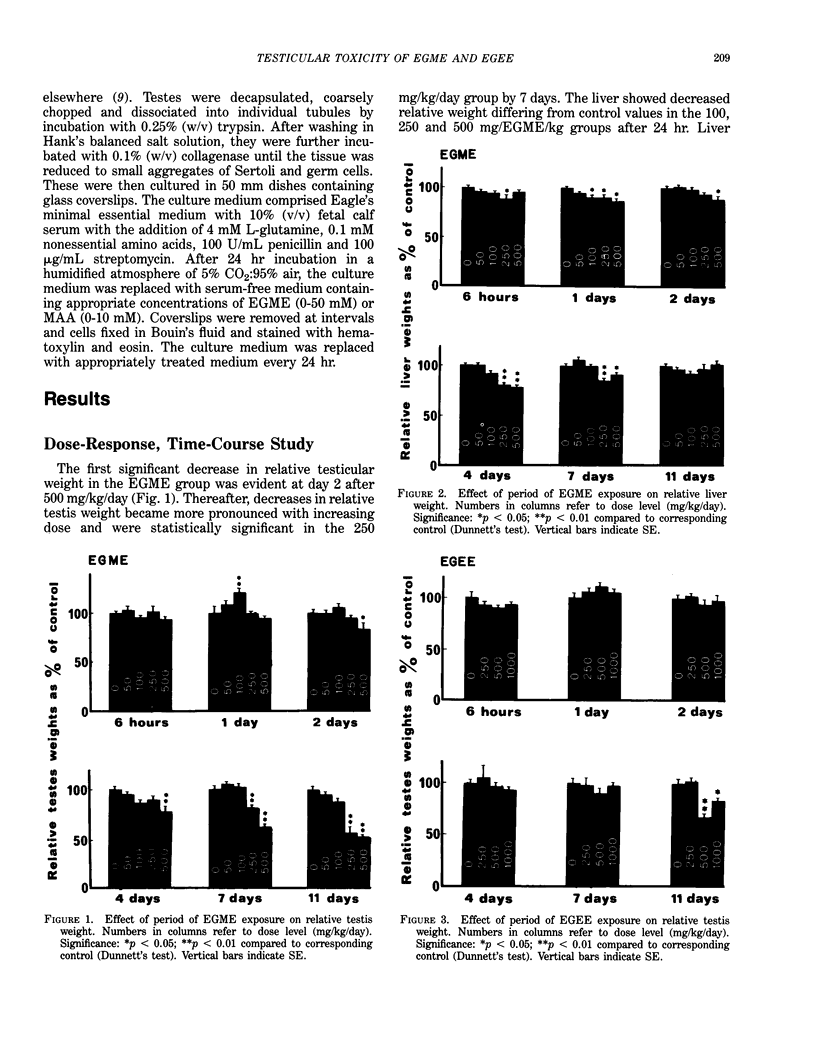
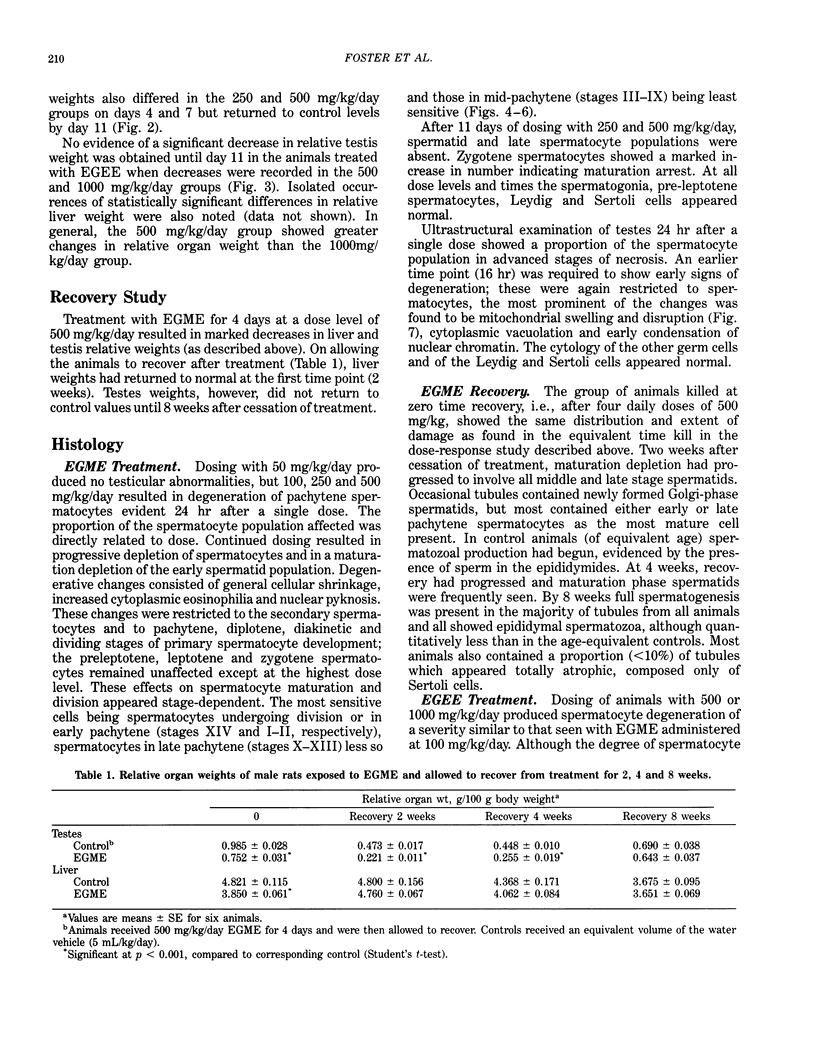
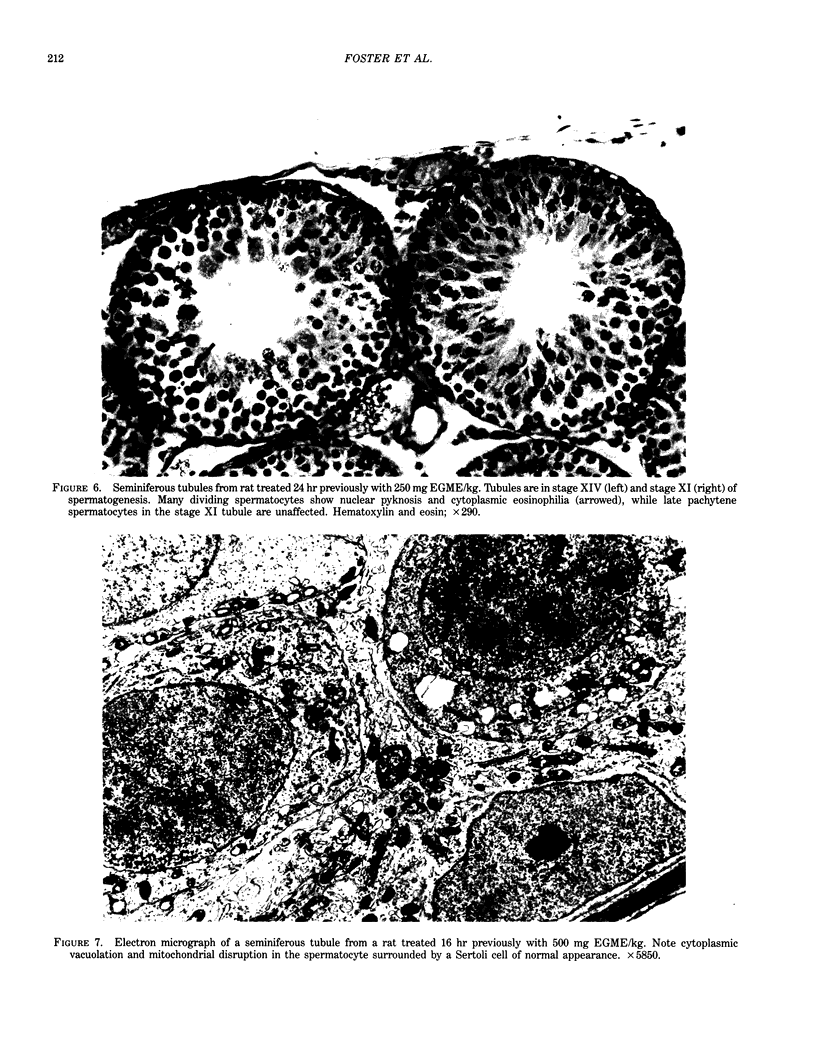
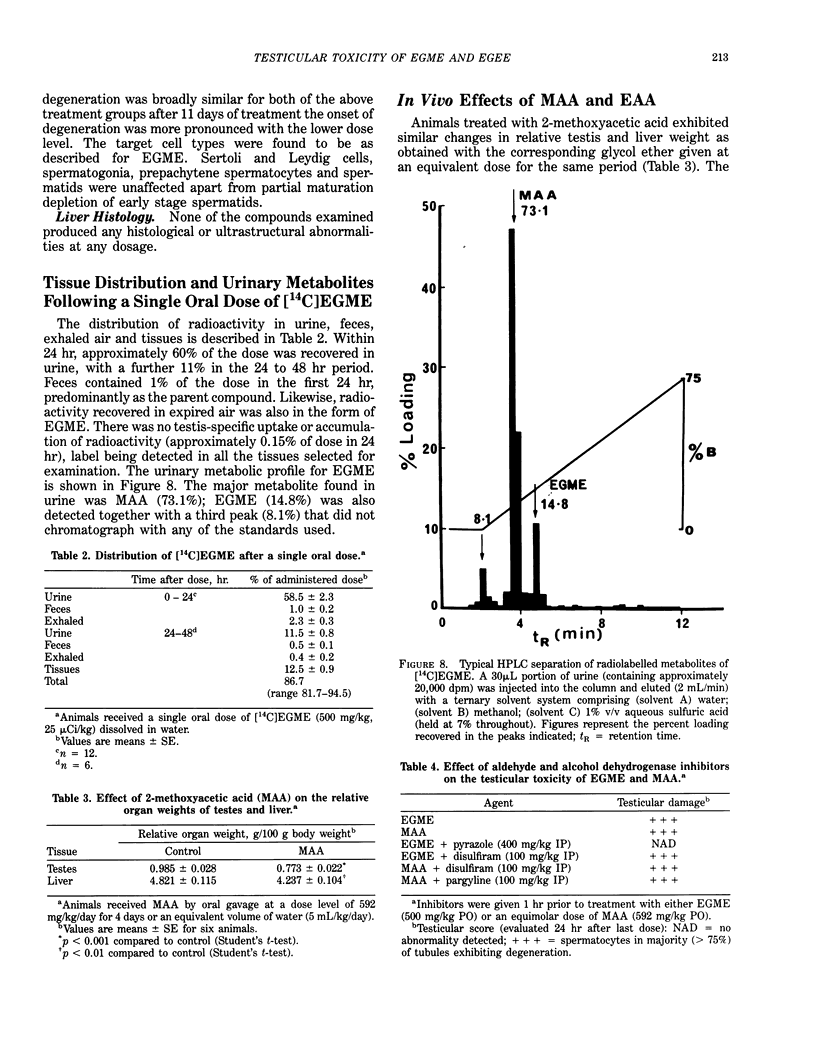
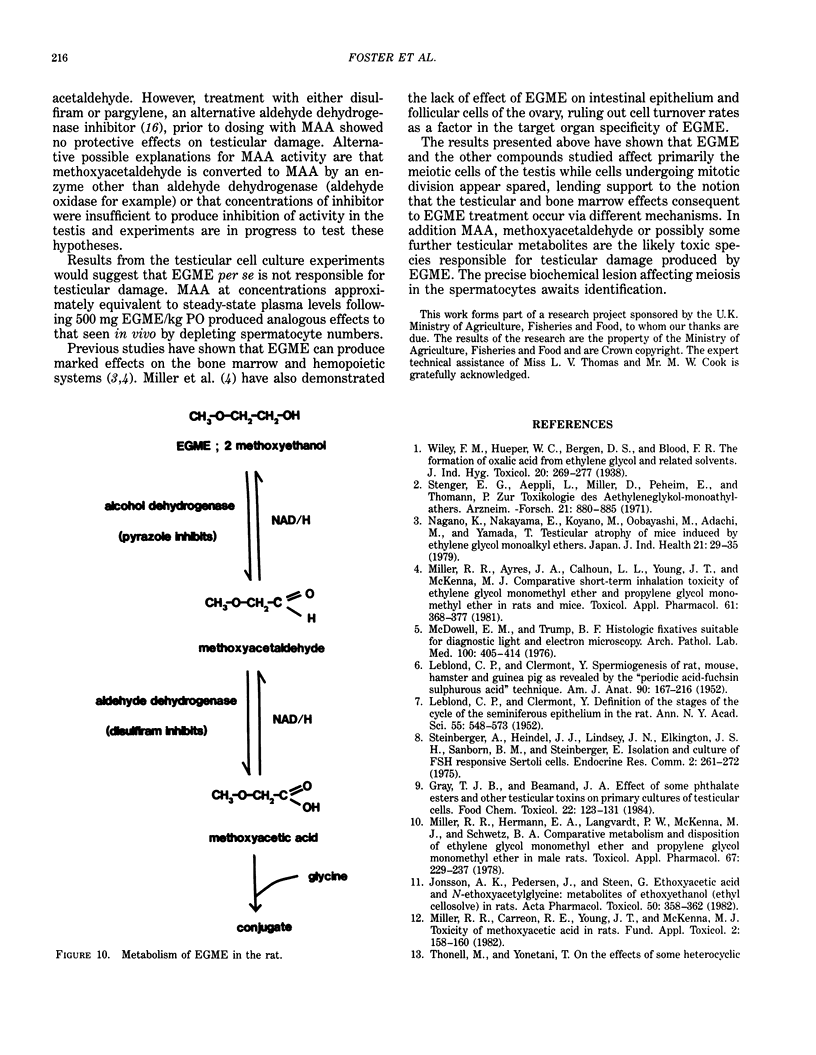
Ethylene glycol monomethyl ether (EGME) and ethylene glycol monoethyl ether (EGEE) were administered orally to young male rats at doses varying from 50 to 500 mg/kg/day and 250 to 1000 mg/kg/day for EGME and EGEE, respectively, for 11 days. At sequential times animals were killed and testicular histology examined. The initial and major site of damage following EGME treatment was restricted to the primary spermatocytes undergoing postzygotene meiotic maturation and division. EGEE produced damage of an identical nature, but a larger dose was required to elicit equivalent severity (500 mg EGEE/kg being approximately equivalent to 100 mg EGME/kg). Additionally, within the spermatocyte population, differential sensitivity was observed depending on the precise stage of meiotic maturation: dividing (stage XIV) and early pachytene (stages I-II) greater than late pachytene (stages VIII-XIII) greater than mid-pachytene (stages III-VII). Equivalent doses of methoxyacetic acid (MAA) and ethoxyacetic acid (EAA) gave injury similar to the corresponding glycol ether. When animals were pretreated with inhibitors of alcohol metabolism followed by a testicular toxic dose of EGME (500 mg/kg), an inhibitor of alcohol dehydrogenase (pyrazole) offered complete protection. Pretreatment with the aldehyde dehydrogenase inhibitors disulfiram or pargyline did not ameliorate the testicular toxicity of EGME. In mixed cultures of Sertoli-germ cells, MAA and not EGME produced effects on spermatocytes analogous to that seen in vivo, at concentrations approximately equivalent to steady-state plasma levels after a single oral dose of EGME (500 mg/kg). It would seem likely that a metabolite (MAA or possibly methoxyacetaldehyde) and not EGME is responsible for the production of testicular damage.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cederbaum A. I., Dicker E. The effect of pargyline on the metabolism of ethanol and acetaldehyde by isolated rat liver cells. Arch Biochem Biophys. 1979 Apr 1;193(2):551–559. doi: 10.1016/0003-9861(79)90062-6. [DOI] [PubMed] [Google Scholar]
- Gray T. J., Beamand J. A. Effect of some phthalate esters and other testicular toxins on primary cultures of testicular cells. Food Chem Toxicol. 1984 Feb;22(2):123–131. doi: 10.1016/0278-6915(84)90092-9. [DOI] [PubMed] [Google Scholar]
- Jönsson A. K., Pedersen J., Steen G. Ethoxyacetic acid and N-ethoxyacetylglycine: metabolites of ethoxyethanol (ethylcellosolve) in rats. Acta Pharmacol Toxicol (Copenh) 1982 May;50(5):358–362. doi: 10.1111/j.1600-0773.1982.tb00987.x. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., CLERMONT Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952 Nov 20;55(4):548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., CLERMONT Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat. 1952 Mar;90(2):167–215. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- Marchner H., Tottmar O. A comparative study on the effects of disulfiram, cyanamide and 1-aminocyclopropanol on the acetaldehyde metabolism in rats. Acta Pharmacol Toxicol (Copenh) 1978 Sep;43(3):219–232. doi: 10.1111/j.1600-0773.1978.tb02258.x. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Miller R. R., Ayres J. A., Calhoun L. L., Young J. T., McKenna M. J. Comparative short-term inhalation toxicity of ethylene glycol monomethyl ether and propylene glycol monomethyl ether in rats and mouse. Toxicol Appl Pharmacol. 1981 Dec;61(3):368–377. doi: 10.1016/0041-008x(81)90358-6. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Carreon R. E., Young J. T., McKenna M. J. Toxicity of methoxyacetic acid in rats. Fundam Appl Toxicol. 1982 Jul-Aug;2(4):158–160. doi: 10.1016/s0272-0590(82)80039-0. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Hermann E. A., Langvardt P. W., McKenna M. J., Schwetz B. A. Comparative metabolism and disposition of ethylene glycol monomethyl ether and propylene glycol monomethyl ether in male rats. Toxicol Appl Pharmacol. 1983 Feb;67(2):229–237. doi: 10.1016/0041-008x(83)90229-6. [DOI] [PubMed] [Google Scholar]
- Nagano K., Nakayama E., Koyano M., Oobayashi H., Adachi H., Yamada T. [Testicular atrophy of mice induced by ethylene glycol mono alkyl ethers (author's transl)]. Sangyo Igaku. 1979 Jan;21(1):29–35. doi: 10.1539/joh1959.21.29. [DOI] [PubMed] [Google Scholar]
- Steinberger A., Heindel J. J., Lindsey J. N., Elkington J. S., Sanborn B. M., Steinberger E. Isolation and culture of FSH responsive Sertoli cells. Endocr Res Commun. 1975;2(3):261–272. doi: 10.3109/07435807509053853. [DOI] [PubMed] [Google Scholar]
- Stenger E. G., Aeppli L., Müller D., Peheim E., Thomann P. Zur Toxikologie des Athylenglykol-Monoäthyläthers. Arzneimittelforschung. 1971 Jun;21(6):880–885. [PubMed] [Google Scholar]