Abstract
Interest in the olfactory mucosa has increased in recent years, since it has been shown to possess a considerable amount of cytochrome P-450-dependent monooxygenase activity and a wide variety of chemicals have been identified as olfactory toxins. Many chemicals induce lesions of a general nature in the olfactory mucosa, i.e., inflammation, degeneration, regeneration, and proliferation, whereas others cause more specific effects. Changes in the olfactory mucosa with reference to chemicals that initiate them are reviewed in this paper. Studies with 3-trifluoromethyl pyridine (3FMP) illustrate some of these general changes and show the importance of examining the olfactory mucosa at early time periods. The earliest damage seen by light microscopy was 6 hr after a single inhalation exposure to 3FMP, and by day 3, early regenerative changes were observed. Changes were seen by electron microscopy 30 min after an oral dose, and the primary site of toxicity appeared to be the Bowman's glands. Although atrophy of nerve bundles in the lamina propria would be the expected consequence of severe necrosis of the sensory cells, this is not always the case. Exposure to irritants such as acetaldehyde, formaldehyde, and dimethylamine results in nerve bundle atrophy, but with chemicals such as 3FMP, 3-methylindole, and 3-methylfuran--which are activated by mixed-function oxidases--the nerve bundles remain intact. Future work, including metabolism studies, will provide information on the mode of action of these chemicals.
Full text
PDF





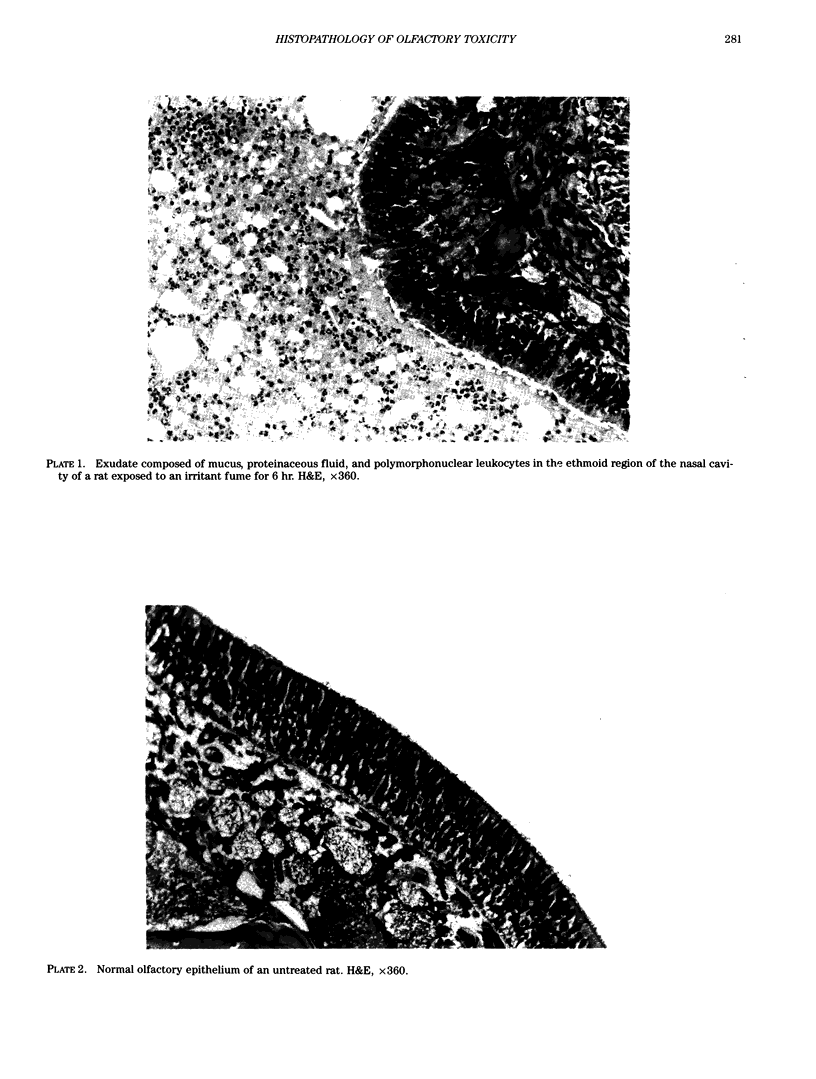








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelman L. M., Woutersen R. A., Feron V. J. Inhalation toxicity of acetaldehyde in rats. I. Acute and subacute studies. Toxicology. 1982;23(4):293–307. doi: 10.1016/0300-483x(82)90068-3. [DOI] [PubMed] [Google Scholar]
- Benson J. M., Burt D. G., Carpenter R. L., Eidson A. F., Hahn F. F., Haley P. J., Hanson R. L., Hobbs C. H., Pickrell J. A., Dunnick J. K. Comparative inhalation toxicity of nickel sulfate to F344/N rats and B6C3F1 mice exposed for twelve days. Fundam Appl Toxicol. 1988 Jan;10(1):164–178. doi: 10.1016/0272-0590(88)90261-8. [DOI] [PubMed] [Google Scholar]
- Benson J. M., Carpenter R. L., Hahn F. F., Haley P. J., Hanson R. L., Hobbs C. H., Pickrell J. A., Dunnick J. K. Comparative inhalation toxicity of nickel subsulfide to F344/N rats and B6C3F1 mice exposed for 12 days. Fundam Appl Toxicol. 1987 Aug;9(2):251–265. doi: 10.1016/0272-0590(87)90047-9. [DOI] [PubMed] [Google Scholar]
- Brittebo E. B. Metabolism of progesterone by the nasal mucosa in mice and rats. Acta Pharmacol Toxicol (Copenh) 1982 Nov;51(5):441–445. doi: 10.1111/j.1600-0773.1982.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Brittebo E. B. N-Demethylation of aminopyrine by the nasal mucosa in mice and rats. Acta Pharmacol Toxicol (Copenh) 1982 Sep;51(3):227–232. doi: 10.1111/j.1600-0773.1982.tb01018.x. [DOI] [PubMed] [Google Scholar]
- Brittebo E., Brandt I. Metabolism of chlorobenzene in the mucosa of the murine respiratory tract. Lung. 1984;162(2):79–88. doi: 10.1007/BF02715634. [DOI] [PubMed] [Google Scholar]
- Buckley L. A., Morgan K. T., Swenberg J. A., James R. A., Hamm T. E., Jr, Barrow C. S. The toxicity of dimethylamine in F-344 rats and B6C3F1 mice following a 1-year inhalation exposure. Fundam Appl Toxicol. 1985 Apr;5(2):341–352. doi: 10.1016/0272-0590(85)90082-x. [DOI] [PubMed] [Google Scholar]
- Dahl A. R., Hadley W. M., Hahn F. F., Benson J. M., McClellan R. O. Cytochrome P-450-dependent monooxygenases in olfactory epithelium of dogs: possible role in tumorigenicity. Science. 1982 Apr 2;216(4541):57–59. doi: 10.1126/science.7063870. [DOI] [PubMed] [Google Scholar]
- Feron V. J., Kroes R. One-year time-sequence inhalation toxicity study of vinyl chloride in rats. II. Morphological changes in the respiratory tract, ceruminous glands, brain, kidneys, heart and spleen. Toxicology. 1979 Jun-Jul;13(2):131–141. [PubMed] [Google Scholar]
- Feron V. J., Kruysse A., Dreefvan der Meulen H. C. Repeated exposure to furfural vapour: 13-week study in Syrian golden hamsters. Zentralbl Bakteriol B. 1979 Jun;168(5-6):442–451. [PubMed] [Google Scholar]
- Feron V. J., Kruysse A. Effects of exposure to furfural vapour in hamsters simultaneously treated with benzo[alpha] pyrene or diethylnitrosamine. Toxicology. 1978 Oct;11(2):127–144. doi: 10.1016/s0300-483x(78)90889-2. [DOI] [PubMed] [Google Scholar]
- Feron V. J., Kruysse A., Til H. P., Immel H. R. Repeated exposure to acrolein vapour: subacute studies in hamsters, rats and rabbits. Toxicology. 1978 Feb;9(1-2):47–57. doi: 10.1016/0300-483x(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Gagnaire F., Zissu D., Bonnet P., De Ceaurriz J. Nasal and pulmonary toxicity of allyl glycidyl ether in mice. Toxicol Lett. 1987 Dec;39(2-3):139–145. doi: 10.1016/0378-4274(87)90226-8. [DOI] [PubMed] [Google Scholar]
- Gaskell B. A., Hext P. M., Pigott G. H., Hodge M. C., Tinston D. J. Olfactory and hepatic changes following inhalation of 3-trifluoromethyl pyridine in rats. Toxicology. 1988 Jun;50(1):57–68. doi: 10.1016/0300-483x(88)90121-7. [DOI] [PubMed] [Google Scholar]
- Graziadei G. A., Graziadei P. P. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979 Apr;8(2):197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P., Graziadei G. A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979 Feb;8(1):1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Greenblatt M., Rijhsinghani K. Comparative cytopathologic alterations induced by alkylnitrosamines in nasal epithelium of the Syrian hamster. J Natl Cancer Inst. 1969 Mar;42(3):421–433. [PubMed] [Google Scholar]
- HERROLD K. M. EFFECT OF ROUTE OF ADMINISTRATION ON THE CARCINOGENIC ACTION OF DIETHYLNITROSAMINE (N-NITROSODIETHYLAMINE). Br J Cancer. 1964 Dec;18:763–767. doi: 10.1038/bjc.1964.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERROLD K. M. INDUCTION OF OLFACTORY NEUROEPITHELIAL TUMORS IN SYRIAN HAMSTERS BY DIETHYLNITROSAMINE. Cancer. 1964 Jan;17:114–121. doi: 10.1002/1097-0142(196401)17:1<114::aid-cncr2820170115>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Hadley W. M., Dahl A. R. Cytochrome P-450 dependent monooxygenase activity in rat nasal epithelial membranes. Toxicol Lett. 1982 Mar;10(4):417–422. doi: 10.1016/0378-4274(82)90240-5. [DOI] [PubMed] [Google Scholar]
- Haschek W. M., Boyd M. R., Hakkinen P. J., Owenby C. S., Witschi H. Acute inhalation toxicity of 3-methylfuran in the mouse: pathology, cell kinetics, and respiratory rate effects. Toxicol Appl Pharmacol. 1984 Jan;72(1):124–133. doi: 10.1016/0041-008x(84)90256-4. [DOI] [PubMed] [Google Scholar]
- Hurtt M. E., Morgan K. T., Working P. K. Histopathology of acute toxic responses in selected tissues from rats exposed by inhalation to methyl bromide. Fundam Appl Toxicol. 1987 Aug;9(2):352–365. doi: 10.1016/0272-0590(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Jiang X. Z., Buckley L. A., Morgan K. T. Pathology of toxic responses to the RD50 concentration of chlorine gas in the nasal passages of rats and mice. Toxicol Appl Pharmacol. 1983 Nov;71(2):225–236. doi: 10.1016/0041-008x(83)90339-3. [DOI] [PubMed] [Google Scholar]
- Kerns W. D., Pavkov K. L., Donofrio D. J., Gralla E. J., Swenberg J. A. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 1983 Sep;43(9):4382–4392. [PubMed] [Google Scholar]
- Lee K. P., Trochimowicz H. J. Induction of nasal tumors in rats exposed to hexamethylphosphoramide by inhalation. J Natl Cancer Inst. 1982 Jan;68(1):157–171. [PubMed] [Google Scholar]
- Miller R. R., Ayres J. A., Jersey G. C., McKenna M. J. Inhalation toxicity of acrylic acid. Fundam Appl Toxicol. 1981 May-Jun;1(3):271–277. doi: 10.1016/s0272-0590(81)80127-3. [DOI] [PubMed] [Google Scholar]
- Miller R. R., Young J. T., Kociba R. J., Keyes D. G., Bodner K. M., Calhoun L. L., Ayres J. A. Chronic toxicity and oncogenicity bioassay of inhaled ethyl acrylate in Fischer 344 rats and B6C3F1 mice. Drug Chem Toxicol. 1985;8(1-2):1–42. doi: 10.3109/01480548509011632. [DOI] [PubMed] [Google Scholar]
- Morse C. C., Boyd M. R., Witschi H. The effect of 3-methylfuran inhalation exposure on the rat nasal cavity. Toxicology. 1984 Apr 2;30(3):195–204. doi: 10.1016/0300-483x(84)90091-x. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Grandjean C. J., Knepper S. Selective induction of nasal cavity tumors in rats by diallylnitrosamine. J Cancer Res Clin Oncol. 1985;109(1):5–8. doi: 10.1007/BF01884247. [DOI] [PubMed] [Google Scholar]
- Pour P., Cardesa A., Althoff J., Mohr U. Tumorigenesis in the nasal olfactory region of Syrian golden hamsters as a result of di-n-propylnitrosamine and related compounds. Cancer Res. 1974 Jan;34(1):16–26. [PubMed] [Google Scholar]
- Reznik G., Reznik-Schüller H., Ward J. M., Stinson S. F. Morphology of nasal-cavity tumours in rats after chronic inhalation of 1,2-dibromo-3-chloropropane. Br J Cancer. 1980 Nov;42(5):772–781. doi: 10.1038/bjc.1980.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik G., Stinson S. F., Ward J. M. Respiratory pathology in rats and mice after inhalation of 1,2-dibromo-3-chloropropane or 1,2 dibromoethane for 13 weeks. Arch Toxicol. 1980 Dec;46(3-4):233–240. doi: 10.1007/BF00310439. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Takano T., Shirai T., Suzuki M., Baba S., Ito N. Comparative light and scanning electron microscopic observations of nasal cavity carcinogenesis in rats treated with 1,4-dinitrosopiperazine. J Cancer Res Clin Oncol. 1984;108(2):186–191. doi: 10.1007/BF00402465. [DOI] [PubMed] [Google Scholar]
- Tucker M. J. Carcinogenic action of quinoxaline 1,4-dioxide in rats. J Natl Cancer Inst. 1975 Jul;55(1):137–146. doi: 10.1093/jnci/55.1.137. [DOI] [PubMed] [Google Scholar]
- Turk M. A., Henk W. G., Flory W. 3-Methylindole-induced nasal mucosal damage in mice. Vet Pathol. 1987 Sep;24(5):400–403. doi: 10.1177/030098588702400506. [DOI] [PubMed] [Google Scholar]
- Woutersen R. A., Appelman L. M., Feron V. J., Van der Heijden C. A. Inhalation toxicity of acetaldehyde in rats. II. Carcinogenicity study: interim results after 15 months. Toxicology. 1984 May 14;31(2):123–133. doi: 10.1016/0300-483x(84)90004-0. [DOI] [PubMed] [Google Scholar]



















