Abstract
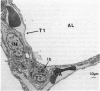
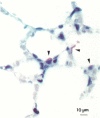
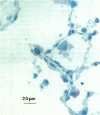
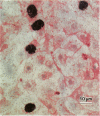
Alveolar Type II cells serve two major functions in the lung, both of which are essential for the preservation of normal lung function. First, Type II cells synthesize and secrete pulmonary surfactant, and second, they function as progenitor cells for maintaining the alveolar epithelium. The Type II cell population of the lung is quite sensitive to the deposition of toxicants in the distal lung, responding in two principal ways. Damage to the Type I epithelium stimulates Type II cells to proliferate and subsequently differentiate to replace the injured Type I cells. Second, a portion of the Type II cell population may become hypertrophic. Both of these events are frequent findings in the diseased or damaged lung. The Type II cell changes are often associated with increases in surfactant pools. In those cases where ultrastructural characteristics of hypertrophic Type II cells were examined, the appearance of these cells was consistent with that of an activated cell type. Alterations in the lamellar body compartment are a common finding in hypertrophic Type II cells, with increases in both lamellar body size and number. It is likely that the hypertrophic, or activated, Type II cells account for the increased levels of surfactant found in the lungs after exposure to a variety of toxic agents. We examined, in detail, Type II cell hyperplasia and hypertrophy induced by silica deposition. Both Type II cell hyperplasia and hypertrophy were prominent responses. The proliferative response led to an approximate doubling of the number of Type II cells in the lung.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H., Cote M. G., Witschi H. Lung injury induced by butylated hydroxytoluene: cytodynamic and biochemical studies in mice. Lab Invest. 1977 Jan;36(1):26–32. [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest. 1975 Jun;32(6):736–745. [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Response of mouse lung to crocidolite asbestos. 1. Minimal fibrotic reaction to short fibres. J Pathol. 1987 Jun;152(2):99–107. doi: 10.1002/path.1711520206. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Response of mouse lung to crocidolite asbestos. 2. Pulmonary fibrosis after long fibres. J Pathol. 1987 Jun;152(2):109–117. doi: 10.1002/path.1711520207. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. Role of polymorphonuclear leukocytes in silica-induced pulmonary fibrosis. Am J Pathol. 1984 Oct;117(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Adamson I. Y., Young L., Bowden D. H. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol. 1988 Feb;130(2):377–383. [PMC free article] [PubMed] [Google Scholar]
- Amanuma K., Suzuki K. T. Effect of intratracheal instillation of cadmium chloride on phospholipids in alveolar wash fluid. Toxicology. 1987 Jun;44(3):321–328. doi: 10.1016/0300-483x(87)90033-3. [DOI] [PubMed] [Google Scholar]
- Aso Y., Yoneda K., Kikkawa Y. Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab Invest. 1976 Dec;35(6):558–568. [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Haller E. M., Shelley S. A., Montgomery M. R. Ozone-induced lamellar body responses in a rat model for alveolar injury and repair. Am J Pathol. 1988 Aug;132(2):330–344. [PMC free article] [PubMed] [Google Scholar]
- Barry B. E., Crapo J. D. Application of morphometric methods to study diffuse and focal injury in the lung caused by toxic agents. Crit Rev Toxicol. 1985;14(1):1–32. doi: 10.3109/10408448509023763. [DOI] [PubMed] [Google Scholar]
- Barry B. E., Wong K. C., Brody A. R., Crapo J. D. Reaction of rat lungs to inhaled chrysotile asbestos following acute and subchronic exposures. Exp Lung Res. 1983 Jul;5(1):1–21. doi: 10.3109/01902148309061501. [DOI] [PubMed] [Google Scholar]
- Castranova V., Rabovsky J., Tucker J. H., Miles P. R. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol Appl Pharmacol. 1988 May;93(3):472–483. doi: 10.1016/0041-008x(88)90051-8. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Peters-Golden M., Marsh-Salin J., Shelburne J. S. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest. 1978 Dec;39(6):640–653. [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Brody A. R., Hook G. E. Induction of intra- and extra-cellular phospholipids in the lungs of rats exposed to silica. Biochem J. 1986 Jan 1;233(1):111–118. doi: 10.1042/bj2330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Gladen B. C., George G., Chhabra R. S., Hook G. E. Effects of silica on the composition of the pulmonary extracellular lining. Toxicol Appl Pharmacol. 1986 Jun 15;84(1):66–83. doi: 10.1016/0041-008x(86)90417-5. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Hook G. E. The relationship between intra- and extra-cellular surfactant phospholipids in the lungs of rabbits and the effects of silica-induced lung injury. Biochem J. 1986 Oct 1;239(1):59–67. doi: 10.1042/bj2390059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethloff L. A., Gladen B. C., Gilmore L. B., Hook G. E. Quantitation of cellular and extracellular constituents of the pulmonary lining in rats by using bronchoalveolar lavage. Effects of silica-induced pulmonary inflammation. Am Rev Respir Dis. 1987 Oct;136(4):899–907. doi: 10.1164/ajrccm/136.4.899. [DOI] [PubMed] [Google Scholar]
- Emerson R. J., Davis G. S. Effect of alveolar lining material-coated silica on rat alveolar macrophages. Environ Health Perspect. 1983 Sep;51:81–84. doi: 10.1289/ehp.835181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975 Feb;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Fallon J. T. Specific Tissue Reaction to Phospholipids: A Suggested Explanation for the Similarity of the Lesions of Silicosis and Pulmonary Tubercolosis. Can Med Assoc J. 1937 Mar;36(3):223–228. [PMC free article] [PubMed] [Google Scholar]
- Farrell P. M., Avery M. E. Hyaline membrane disease. Am Rev Respir Dis. 1975 May;111(5):657–688. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- Faulkner C. S., 2nd, Esterly J. R. Ultrastructural changes in the alveolar epithelium in response to Freund's adjuvant. Am J Pathol. 1971 Sep;64(3):559–566. [PMC free article] [PubMed] [Google Scholar]
- Fringes B., Gorgas K., Reith A. Modification of surfactant metabolizing cells in rat lung by clofibrate, a hypolipidemic peroxisome proliferating agent. Evidence to suggest that clofibrate influences pulmonary surfactant metabolism. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54(4):232–240. doi: 10.1007/BF02899216. [DOI] [PubMed] [Google Scholar]
- Gabor S., Zugravu E., Kováts A., Böhm B., Andrasoni D. Effects of quartz on lung surfactant. Environ Res. 1978 Jul;16(1-3):443–448. doi: 10.1016/0013-9351(78)90177-9. [DOI] [PubMed] [Google Scholar]
- Gottschall J. L., Walzer P. D., Yoneda K. The morphologic changes of the rat type II pneumocytes induced by oxytetracycline. Lab Invest. 1979 Jul;41(1):5–12. [PubMed] [Google Scholar]
- Haies D. M., Gil J., Weibel E. R. Morphometric study of rat lung cells. I. Numerical and dimensional characteristics of parenchymal cell population. Am Rev Respir Dis. 1981 May;123(5):533–541. doi: 10.1164/arrd.1981.123.5.533. [DOI] [PubMed] [Google Scholar]
- Harwood J. L., Desai R., Hext P., Tetley T., Richards R. Characterization of pulmonary surfactant from ox, rabbit, rat and sheep. Biochem J. 1975 Dec;151(3):707–714. doi: 10.1042/bj1510707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppleston A. G., Fletcher K., Wyatt I. Abnormalities of lung lipids following inhalation of quartz. Experientia. 1972 Aug 15;28(8):938–939. doi: 10.1007/BF01924959. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Fletcher K., Wyatt I. Changes in the composition of lung lipids and the "turnover" of dipalmitoyl lecithin in experimental alveolar lipo-proteinosis induced by inhaled quartz. Br J Exp Pathol. 1974 Aug;55(4):384–395. [PMC free article] [PubMed] [Google Scholar]
- Heppleston A. G., Wright N. A., Stewart J. A. Experimental alveolar lipo-proteinosis following the inhalation of silica. J Pathol. 1970 Aug;101(4):293–307. doi: 10.1002/path.1711010402. [DOI] [PubMed] [Google Scholar]
- Holm B. A., Notter R. H., Leary J. F., Matalon S. Alveolar epithelial changes in rabbits after a 21-day exposure to 60% O2. J Appl Physiol (1985) 1987 Jun;62(6):2230–2236. doi: 10.1152/jappl.1987.62.6.2230. [DOI] [PubMed] [Google Scholar]
- Hook G. E. Extracellular hydrolases of the lung. Biochemistry. 1978 Feb 7;17(3):520–528. doi: 10.1021/bi00596a023. [DOI] [PubMed] [Google Scholar]
- Hook G. E., Gilmore L. B., Talley F. A. Dissolution and reassembly of tubular myelin-like multilamellated structures from the lungs of patients with pulmonary alveolar proteinosis. Lab Invest. 1986 Aug;55(2):194–208. [PubMed] [Google Scholar]
- Hook G. E., Gilmore L. B., Talley F. A. Multilamelled structures from the lungs of patients with pulmonary alveolar proteinosis. Lab Invest. 1984 Jun;50(6):711–725. [PubMed] [Google Scholar]
- Kuhn C., 3rd Cytochemistry of pulmonary alveolar epithelial cells. Am J Pathol. 1968 Nov;53(5):809–833. [PMC free article] [PubMed] [Google Scholar]
- Kumar R. K., Watkins S. G., Lykke A. W. Pulmonary responses to bleomycin-induced injury: an immunomorphologic and electron microscopic study. Exp Pathol. 1985;28(1):33–43. doi: 10.1016/s0232-1513(85)80030-x. [DOI] [PubMed] [Google Scholar]
- Le Mesurier S. M., Lykke A. W., Stewart B. W. Reduced yield of pulmonary surfactant: patterns of response following administration of chemicals to rats by inhalation. Toxicol Lett. 1980 Jan;5(1):89–93. doi: 10.1016/0378-4274(80)90153-8. [DOI] [PubMed] [Google Scholar]
- Le Mesurier S. M., Stewart B. W., Lykke A. W. Injury to type-2 pneumocytes in rats exposed to cigarette smoke. Environ Res. 1981 Feb;24(1):207–217. doi: 10.1016/0013-9351(81)90146-8. [DOI] [PubMed] [Google Scholar]
- Lewis R. W., Harwood J. L., Richards R. J. The fate of instilled pulmonary surfactant in normal and quartz-treated rats. Biochem J. 1987 May 1;243(3):679–685. doi: 10.1042/bj2430679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. M., Witschi H. P. Cadmium-induced lung injury: cell kinetics and long-term effects. Toxicol Appl Pharmacol. 1985 Sep 15;80(2):215–227. doi: 10.1016/0041-008x(85)90078-x. [DOI] [PubMed] [Google Scholar]
- McDermott M., Wagner J. C., Tetley T., Harwood J., Richards R. J. The effects of inhaled silica and chrysotile on the elastic properties of rat lungs; physiological, physical and biochemical studies of lung surfactant. Inhaled Part. 1975 Sep;4(Pt 2):415–427. [PubMed] [Google Scholar]
- Miller B. E., Chapin R. E., Pinkerton K. E., Gilmore L. B., Maronpot R. R., Hook G. E. Quantitation of silica-induced type II cell hyperplasia by using alkaline phosphatase histochemistry in glycol methacrylate embedded lung. Exp Lung Res. 1987;12(2):135–148. doi: 10.3109/01902148709062837. [DOI] [PubMed] [Google Scholar]
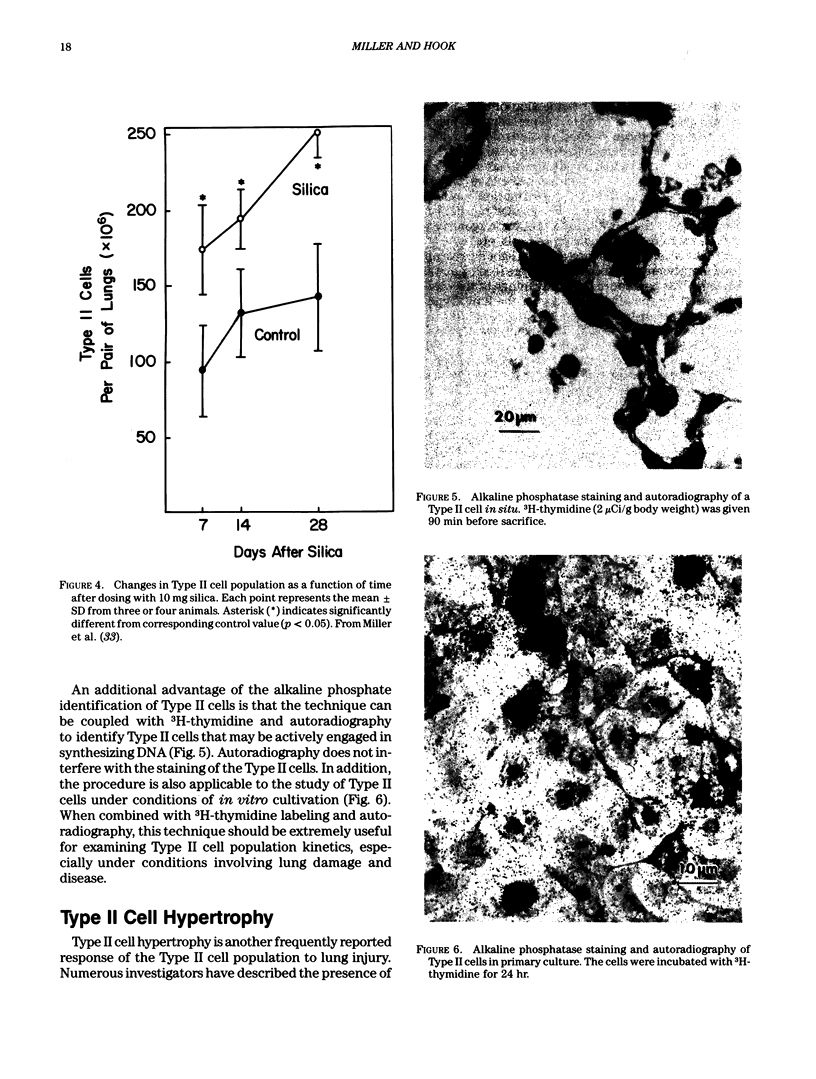
- Miller B. E., Dethloff L. A., Gladen B. C., Hook G. E. Progression of type II cell hypertrophy and hyperplasia during silica-induced pulmonary inflammation. Lab Invest. 1987 Nov;57(5):546–554. [PubMed] [Google Scholar]
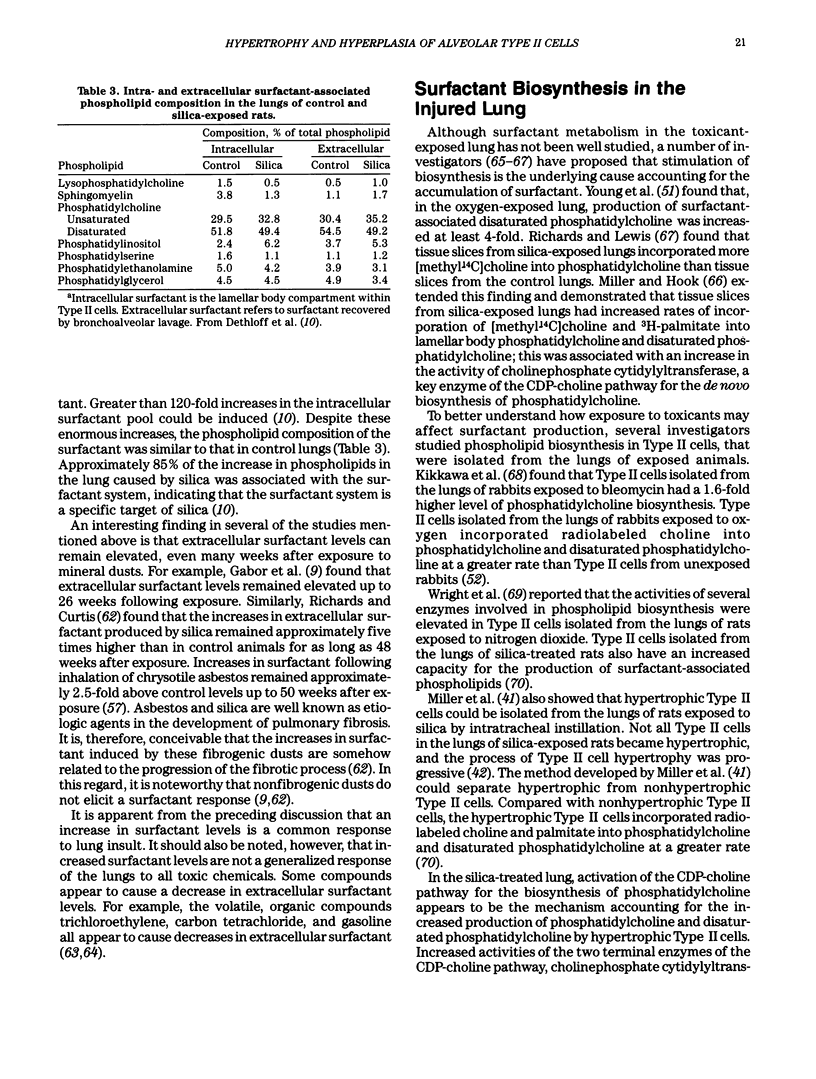
- Miller B. E., Dethloff L. A., Hook G. E. Silica-induced hypertrophy of type II cells in the lungs of rats. Lab Invest. 1986 Aug;55(2):153–163. [PubMed] [Google Scholar]
- Miller B. E., Hook G. E. Isolation and characterization of hypertrophic type II cells from the lungs of silica-treated rats. Lab Invest. 1988 May;58(5):565–575. [PubMed] [Google Scholar]
- Miller B. E., Hook G. E. Stimulation of surfactant phospholipid biosynthesis in the lungs of rats treated with silica. Biochem J. 1988 Aug 1;253(3):659–665. doi: 10.1042/bj2530659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton K. E., Pratt P. C., Brody A. R., Crapo J. D. Fiber localization and its relationship to lung reaction in rats after chronic inhalation of chrysotile asbestos. Am J Pathol. 1984 Dec;117(3):484–498. [PMC free article] [PubMed] [Google Scholar]
- Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988 Oct;138(4):990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- Richards R. J., Curtis C. G. Biochemical and cellular mechanisms of dust-induced lung fibrosis. Environ Health Perspect. 1984 Apr;55:393–416. doi: 10.1289/ehp.8455393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. J., Lewis R. Surfactant changes in experimentally induced disease. Biochem Soc Trans. 1985 Dec;13(6):1084–1087. doi: 10.1042/bst0131084. [DOI] [PubMed] [Google Scholar]
- Robinson P. C., Watters L. C., King T. E., Mason R. J. Idiopathic pulmonary fibrosis. Abnormalities in bronchoalveolar lavage fluid phospholipids. Am Rev Respir Dis. 1988 Mar;137(3):585–591. doi: 10.1164/ajrccm/137.3.585. [DOI] [PubMed] [Google Scholar]
- Rooney S. A. The surfactant system and lung phospholipid biochemistry. Am Rev Respir Dis. 1985 Mar;131(3):439–460. doi: 10.1164/arrd.1985.131.3.439. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Forkert P. G., Oulton M., Rasmusson M. G., Temple S., Fraser M. O., Whitefield S. Pulmonary toxicity of trichloroethylene: induction of changes in surfactant phospholipids and phospholipase A2 activity in the mouse lung. Exp Mol Pathol. 1988 Aug;49(1):141–150. doi: 10.1016/0014-4800(88)90028-7. [DOI] [PubMed] [Google Scholar]
- Sorokin S P. A morphologic and cytochemical study on the great alveolar cell. J Histochem Cytochem. 1966 Dec;14(12):884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Tetley T. D., Richards R. J., Harwood J. L. Changes in pulmonary surfactant and phosphatidylcholine metabolism in rats exposed to chrysotile asbestos dust. Biochem J. 1977 Sep 15;166(3):323–329. doi: 10.1042/bj1660323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thet L. A., Parra S. C., Shelburne J. D. Repair of oxygen-induced lung injury in adult rats. The role of ornithine decarboxylase and polyamines. Am Rev Respir Dis. 1984 Jan;129(1):174–181. doi: 10.1164/arrd.1984.129.1.174. [DOI] [PubMed] [Google Scholar]
- Thet L. A., Parra S. C., Shelburne J. D. Sequential changes in lung morphology during the repair of acute oxygen-induced lung injury in adult rats. Exp Lung Res. 1986;11(3):209–228. doi: 10.3109/01902148609064297. [DOI] [PubMed] [Google Scholar]
- Tryka A. F., Witschi H., Gosslee D. G., McArthur A. H., Clapp N. K. Patterns of cell proliferation during recovery from oxygen injury. Species differences. Am Rev Respir Dis. 1986 Jun;133(6):1055–1059. doi: 10.1164/arrd.1986.133.6.1055. [DOI] [PubMed] [Google Scholar]
- Weaver T. E. Pulmonary surfactant-associated proteins. Gen Pharmacol. 1988;19(3):361–368. doi: 10.1016/0306-3623(88)90029-8. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. A note on differentiation and divisibility of alveolar epithelial cells. Chest. 1974 Apr;65(Suppl):19S–21S. doi: 10.1378/chest.65.4_supplement.19s. [DOI] [PubMed] [Google Scholar]
- Williams R. A., Rhoades R. A., Adams W. S. The response of lung tissue and surfactant to nitrogen dioxide exposure. Arch Intern Med. 1971 Jul;128(1):101–108. [PubMed] [Google Scholar]
- Witsch I. H. Proliferation of type II alveolar cells: a review of common responses in toxic lung injury. Toxicology. 1976 Mar;5(3):267–277. doi: 10.1016/0300-483x(76)90046-9. [DOI] [PubMed] [Google Scholar]
- Wright E. S. Effects of short-term exposure to diesel exhaust on lung cell proliferation and phospholipid metabolism. Exp Lung Res. 1986;10(1):39–55. doi: 10.3109/01902148609057502. [DOI] [PubMed] [Google Scholar]
- Wright E. S., Vang M. J., Finkelstein J. N., Mavis R. D. Changes in phospholipid biosynthetic enzymes in type II cells and alveolar macrophages isolated from rat lungs after NO2 exposure. Toxicol Appl Pharmacol. 1982 Nov;66(2):305–311. doi: 10.1016/0041-008x(82)90296-4. [DOI] [PubMed] [Google Scholar]
- Young S. L., Crapo J. D., Kremers S. A., Brumley G. W. Pulmonary surfactant lipid production in oxygen-exposed rat lungs. Lab Invest. 1982 Jun;46(6):570–576. [PubMed] [Google Scholar]
- Young S. L., Kremers S. A., Apple J. S., Crapo J. D., Brumley G. W. Rat lung surfactant kinetics biochemical and morphometric correlation. J Appl Physiol Respir Environ Exerc Physiol. 1981 Aug;51(2):248–253. doi: 10.1152/jappl.1981.51.2.248. [DOI] [PubMed] [Google Scholar]