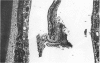Abstract
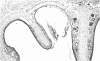
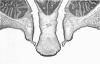
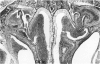
Rodents are commonly used for inhalation toxicology studies, but until recently the nasal passages have often been overlooked or only superficially examined. The rodent nose is a complex organ in which toxicant-induced lesions may vary, depending on the test compound. A working knowledge of rodent nasal anatomy and histology is essential for the proper evaluation of these responses. Lack of a systematic approach for examining rodent nasal tissue has led to a paucity of information regarding nonneoplastic lesions in the rodent nose. Therefore, slides from the National Toxicology Program (NTP) and the Chemical Industry Institute of Toxicology (CIIT) were examined, and the literature was reviewed to assemble the spectrum of nonneoplastic rodent nasal pathology. Presented are lesions associated with the various types of epithelia lining the rodent nasal cavity plus lesions involving accessory nasal structures. Even though there are anatomic and physiologic differences between the rodent and human nose, both rats and mice provide valuable animal models for the study of nasal epithelial toxicity, following administration of chemical compounds.
Full text
PDF





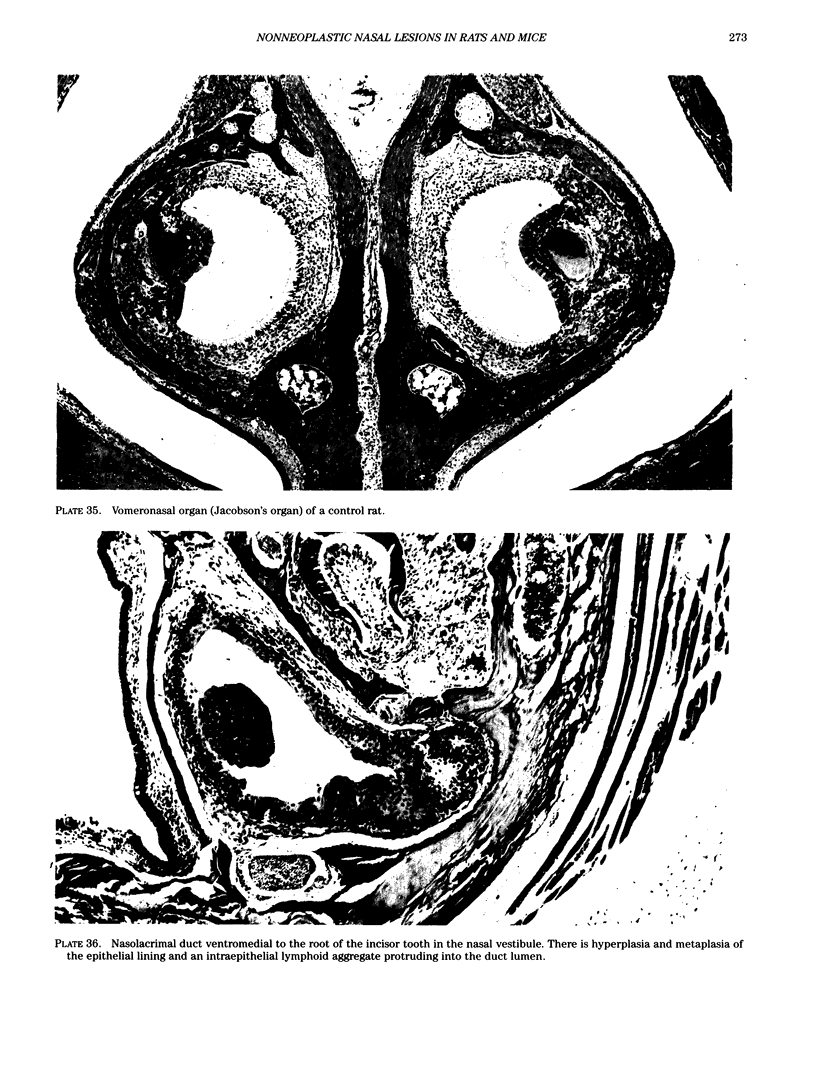
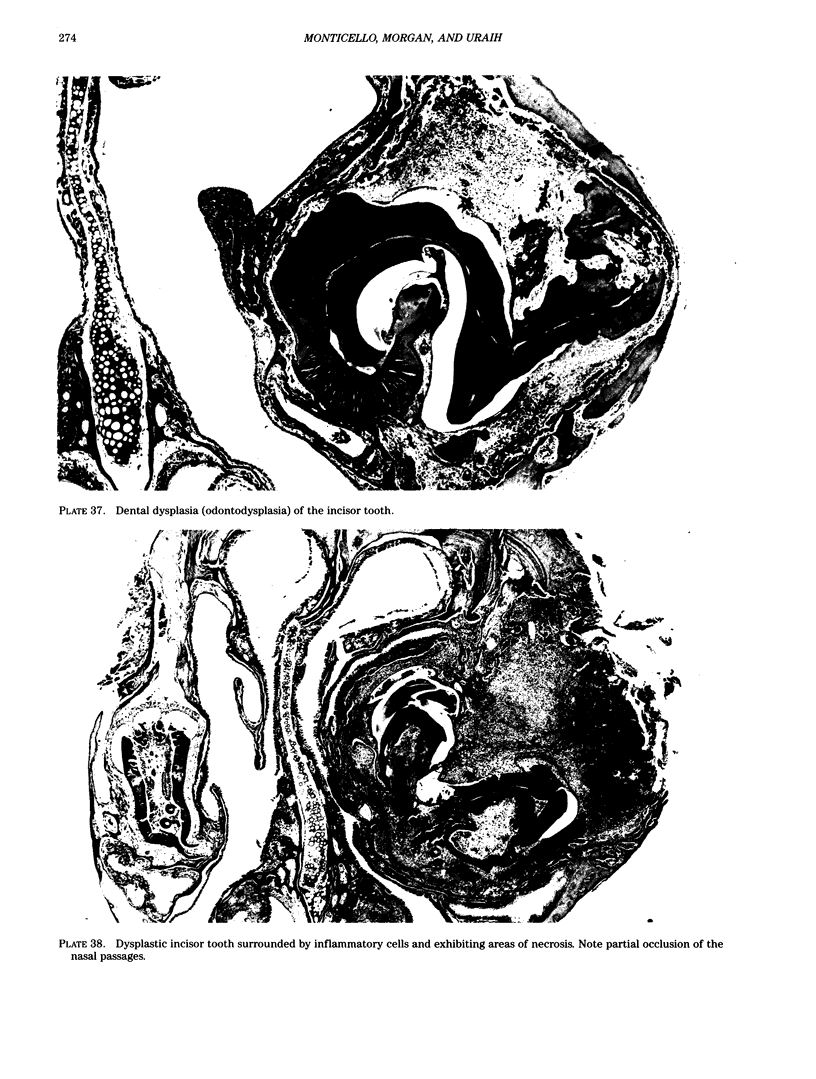
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelman L. M., Woutersen R. A., Feron V. J. Inhalation toxicity of acetaldehyde in rats. I. Acute and subacute studies. Toxicology. 1982;23(4):293–307. doi: 10.1016/0300-483x(82)90068-3. [DOI] [PubMed] [Google Scholar]
- Becci P. J., McDowell E. M., Trump B. F. The respiratory epithelium. IV. Histogenesis of epidermoid metaplasia and carcinoma in situ in the hamster. J Natl Cancer Inst. 1978 Aug;61(2):577–586. [PubMed] [Google Scholar]
- Belinsky S. A., Walker V. E., Maronpot R. R., Swenberg J. A., Anderson M. W. Molecular dosimetry of DNA adduct formation and cell toxicity in rat nasal mucosa following exposure to the tobacco specific nitrosamine 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone and their relationship to induction of neoplasia. Cancer Res. 1987 Nov 15;47(22):6058–6065. [PubMed] [Google Scholar]
- Broderson J. R., Lindsey J. R., Crawford J. E. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol. 1976 Oct;85(1):115–130. [PMC free article] [PubMed] [Google Scholar]
- Buckley L. A., Jiang X. Z., James R. A., Morgan K. T., Barrow C. S. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol Appl Pharmacol. 1984 Jul;74(3):417–429. doi: 10.1016/0041-008x(84)90295-3. [DOI] [PubMed] [Google Scholar]
- Buckley L. A., Morgan K. T., Swenberg J. A., James R. A., Hamm T. E., Jr, Barrow C. S. The toxicity of dimethylamine in F-344 rats and B6C3F1 mice following a 1-year inhalation exposure. Fundam Appl Toxicol. 1985 Apr;5(2):341–352. doi: 10.1016/0272-0590(85)90082-x. [DOI] [PubMed] [Google Scholar]
- Dunnick J. K., Eustis S. L., Piegorsch W. W., Miller R. A. Respiratory tract lesions in F344/N rats and B6C3F1 mice after inhalation exposure to 1,2-epoxybutane. Toxicology. 1988 Jun;50(1):69–82. doi: 10.1016/0300-483x(88)90122-9. [DOI] [PubMed] [Google Scholar]
- Feron V. J., Kruysse A., Til H. P., Immel H. R. Repeated exposure to acrolein vapour: subacute studies in hamsters, rats and rabbits. Toxicology. 1978 Feb;9(1-2):47–57. doi: 10.1016/0300-483x(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Feron V. J., Kruysse A., Woutersen R. A. Respiratory tract tumours in hamsters exposed to acetaldehyde vapour alone or simultaneously to benzo(a)pyrene or diethylnitrosamine. Eur J Cancer Clin Oncol. 1982 Jan;18(1):13–31. doi: 10.1016/0277-5379(82)90020-7. [DOI] [PubMed] [Google Scholar]
- Gaskell B. A., Hext P. M., Pigott G. H., Hodge M. C., Tinston D. J. Olfactory and hepatic changes following inhalation of 3-trifluoromethyl pyridine in rats. Toxicology. 1988 Jun;50(1):57–68. doi: 10.1016/0300-483x(88)90121-7. [DOI] [PubMed] [Google Scholar]
- Giddens W. E., Jr, Fairchild G. A. Effects of sulfur dioxide on the nasal mucosa of mice. Arch Environ Health. 1972 Sep;25(3):166–173. doi: 10.1080/00039896.1972.10666156. [DOI] [PubMed] [Google Scholar]
- Gross E. A., Patterson D. L., Morgan K. T. Effects of acute and chronic dimethylamine exposure on the nasal mucociliary apparatus of F-344 rats. Toxicol Appl Pharmacol. 1987 Sep 30;90(3):359–376. doi: 10.1016/0041-008X(87)90129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschek W. M., Morse C. C., Boyd M. R., Hakkinen P. J., Witschi H. P. Pathology of acute inhalation exposure to 3-methylfuran in the rat and hamster. Exp Mol Pathol. 1983 Dec;39(3):342–354. doi: 10.1016/0014-4800(83)90063-1. [DOI] [PubMed] [Google Scholar]
- Hurtt M. E., Morgan K. T., Working P. K. Histopathology of acute toxic responses in selected tissues from rats exposed by inhalation to methyl bromide. Fundam Appl Toxicol. 1987 Aug;9(2):352–365. doi: 10.1016/0272-0590(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Jiang X. Z., Buckley L. A., Morgan K. T. Pathology of toxic responses to the RD50 concentration of chlorine gas in the nasal passages of rats and mice. Toxicol Appl Pharmacol. 1983 Nov;71(2):225–236. doi: 10.1016/0041-008x(83)90339-3. [DOI] [PubMed] [Google Scholar]
- Katz S., Merzel J. Distribution of epithelia and glands of the nasal septum mucosa in the rat. Acta Anat (Basel) 1977;99(1):58–66. doi: 10.1159/000144835. [DOI] [PubMed] [Google Scholar]
- Kerns W. D., Pavkov K. L., Donofrio D. J., Gralla E. J., Swenberg J. A. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 1983 Sep;43(9):4382–4392. [PubMed] [Google Scholar]
- Klaassen A. B., Kuijpers W., Denucé J. M. Morphological and histochemical aspects of the nasal glands in the rat. Anat Anz. 1981;149(1):51–63. [PubMed] [Google Scholar]
- Klein-Szanto A. J., Boysen M., Reith A. Keratin and involucrin in preneoplastic and neoplastic lesions. Distribution in the nasal mucosa of nickel workers. Arch Pathol Lab Med. 1987 Nov;111(11):1057–1061. [PubMed] [Google Scholar]
- Klein-Szanto A. J., Topping D. C., Heckman C. A., Nettesheim P. Ultrastructural characteristics of carcinogen-induced dysplastic changes in tracheal epithelium. Am J Pathol. 1980 Jan;98(1):83–100. [PMC free article] [PubMed] [Google Scholar]
- Marks S. C., Jr Pathogenesis of osteopetrosis in the ia rat: reduced bone resorption due to reduced osteoclast function. Am J Anat. 1973 Oct;138(2):165–189. doi: 10.1002/aja.1001380204. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N. A., Popp J. A. Ultrastructural characterization of the nasal respiratory epithelium in the rat. Am J Anat. 1984 Jan;169(1):31–43. doi: 10.1002/aja.1001690103. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N. A., Popp J. A. Ultrastructural evaluation of acute nasal toxicity in the rat respiratory epithelium in response to formaldehyde gas. Fundam Appl Toxicol. 1986 Feb;6(2):251–262. [PubMed] [Google Scholar]
- Morgan K. T., Jiang X. Z., Patterson D. L., Gross E. A. The nasal mucociliary apparatus. Correlation of structure and function in the rat. Am Rev Respir Dis. 1984 Aug;130(2):275–281. doi: 10.1164/arrd.1984.130.2.275. [DOI] [PubMed] [Google Scholar]
- Popp J. A., Martin J. T. Surface topography and distribution of cell types in the rat nasal respiratory epithelium: scanning electron microscopic observations. Am J Anat. 1984 Apr;169(4):425–436. doi: 10.1002/aja.1001690405. [DOI] [PubMed] [Google Scholar]
- Reznik G., Reznik-Schüller H., Ward J. M., Stinson S. F. Morphology of nasal-cavity tumours in rats after chronic inhalation of 1,2-dibromo-3-chloropropane. Br J Cancer. 1980 Nov;42(5):772–781. doi: 10.1038/bjc.1980.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik G., Stinson S. F., Ward J. M. Respiratory pathology in rats and mice after inhalation of 1,2-dibromo-3-chloropropane or 1,2 dibromoethane for 13 weeks. Arch Toxicol. 1980 Dec;46(3-4):233–240. doi: 10.1007/BF00310439. [DOI] [PubMed] [Google Scholar]
- Robins M. W., Rowlatt C. Dental abnormalities in aged mice. Gerontologia. 1971;17(5):261–272. doi: 10.1159/000211833. [DOI] [PubMed] [Google Scholar]
- Stewart H. L., Dunn T. B., Snell K. C., Deringer M. K. Tumours of the respiratory tract. IARC Sci Publ. 1979;23:251–287. [PubMed] [Google Scholar]
- Vidić B., Greditzer H. G. The histochemical and microscopical differentiation of the respiratory glands around the maxillary sinus of the rat. Am J Anat. 1971 Dec;132(4):491–513. doi: 10.1002/aja.1001320407. [DOI] [PubMed] [Google Scholar]
- Vidić B., Rana M. W., Bhagat B. D. Reversible damage of rat upper respiratory tract caused by cigarette smoke. Arch Otolaryngol. 1974 Feb;99(2):110–113. doi: 10.1001/archotol.1974.00780030116008. [DOI] [PubMed] [Google Scholar]
- Young J. T. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981 Jul-Aug;1(4):309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]