Abstract
The nasal cavity is susceptible to chemically induced injury as a result of exposure to inhaled irritants. Some responses of the nasal mucosa to inhaled toxicants are species specific. These species-related differences in response may be due to variations in structural, physiologic, and biochemical factors, such as gross nasal cavity structure, distribution of luminal epithelial cell populations along the nasal airway, intranasal airflow patterns, nasal mucociliary apparatus, and nasal xenobiotic metabolism among animal species. This paper reviews the comparative anatomy and irritant-induced pathology of the nasal cavity in laboratory animals. The toxicologist, pathologist, and environmental risk assessor must have a good working knowledge of the similarities and differences in normal nasal structure and response to injury among species before they can select animal models for nasal toxicity studies, recognize toxicant-induced lesions in the nasal airway, and extrapolate experimental results to estimate the possible effects of an inhaled toxicant on the human nasal airway.
Full text
PDF





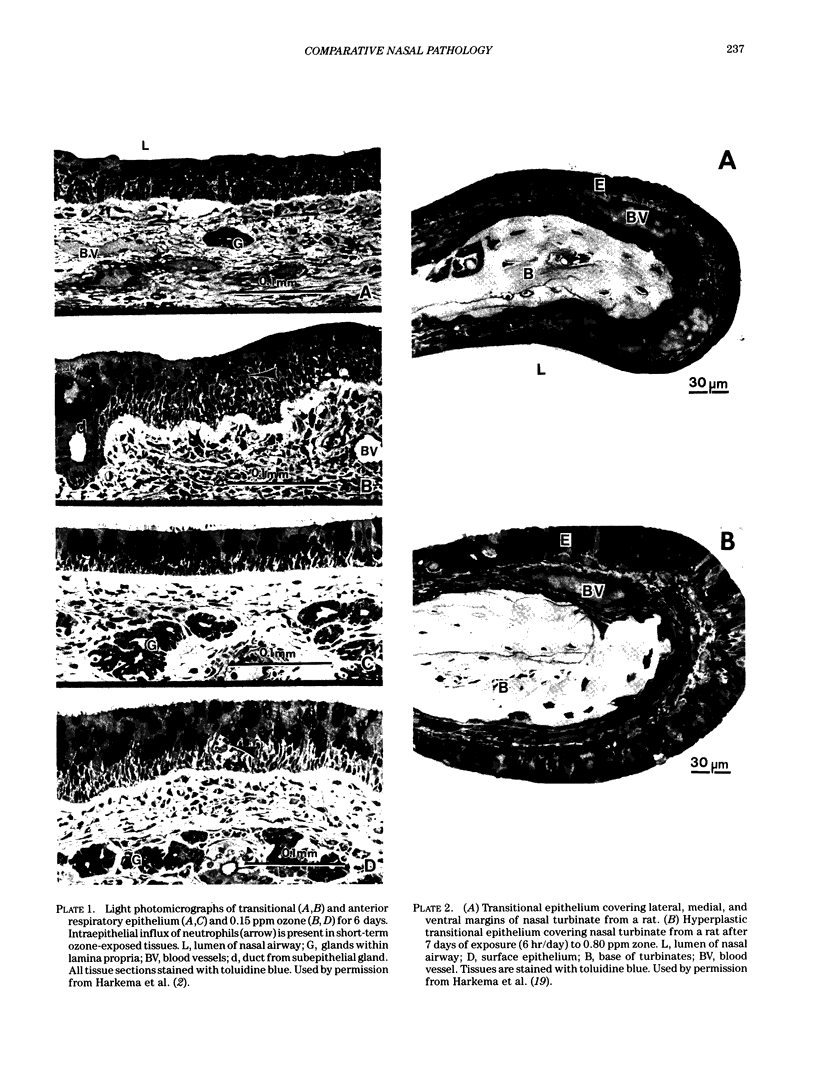
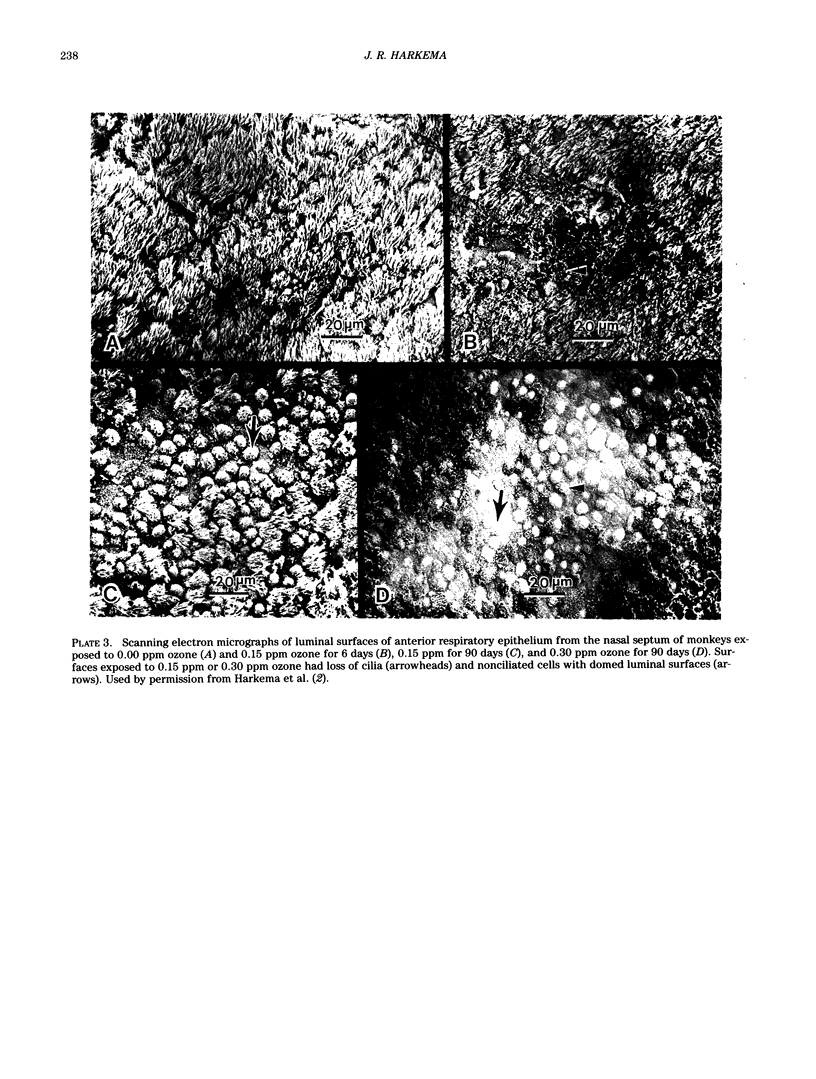
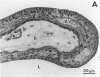
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basrur P. K., Harada T. Alterations in the nasal mucosa of Syrian golden hamsters exposed to cigarette smoke. Prog Exp Tumor Res. 1979;24:283–301. doi: 10.1159/000402105. [DOI] [PubMed] [Google Scholar]
- Bond J. A., Harkema J. R., Russell V. I. Regional distribution of xenobiotic metabolizing enzymes in respiratory airways of dogs. Drug Metab Dispos. 1988 Jan-Feb;16(1):116–124. [PubMed] [Google Scholar]
- Buckley L. A., Jiang X. Z., James R. A., Morgan K. T., Barrow C. S. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol Appl Pharmacol. 1984 Jul;74(3):417–429. doi: 10.1016/0041-008x(84)90295-3. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Gross E. A., Swenberg J. A., Barrow C. S. Nasal cavity deposition, histopathology, and cell proliferation after single or repeated formaldehyde exposures in B6C3F1 mice and F-344 rats. Toxicol Appl Pharmacol. 1983 Apr;68(2):161–176. doi: 10.1016/0041-008x(83)90001-7. [DOI] [PubMed] [Google Scholar]
- Dahl A. R., Hadley W. M., Hahn F. F., Benson J. M., McClellan R. O. Cytochrome P-450-dependent monooxygenases in olfactory epithelium of dogs: possible role in tumorigenicity. Science. 1982 Apr 2;216(4541):57–59. doi: 10.1126/science.7063870. [DOI] [PubMed] [Google Scholar]
- Gaskell B. A. Nonneoplastic changes in the olfactory epithelium--experimental studies. Environ Health Perspect. 1990 Apr;85:275–289. doi: 10.1289/ehp.85-1568318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E. A., Swenberg J. A., Fields S., Popp J. A. Comparative morphometry of the nasal cavity in rats and mice. J Anat. 1982 Aug;135(Pt 1):83–88. [PMC free article] [PubMed] [Google Scholar]
- Hadley W. M., Dahl A. R. Cytochrome P-450-dependent monooxygenase activity in nasal membranes of six species. Drug Metab Dispos. 1983 May-Jun;11(3):275–276. [PubMed] [Google Scholar]
- Harkema J. R., Hotchkiss J. A., Henderson R. F. Effects of 0.12 and 0.80 ppm ozone on rat nasal and nasopharyngeal epithelial mucosubstances: quantitative histochemistry. Toxicol Pathol. 1989;17(3):525–535. doi: 10.1177/019262338901700307. [DOI] [PubMed] [Google Scholar]
- Harkema J. R., Plopper C. G., Hyde D. M., St George J. A., Dungworth D. L. Effects of an ambient level of ozone on primate nasal epithelial mucosubstances. Quantitative histochemistry. Am J Pathol. 1987 Apr;127(1):90–96. [PMC free article] [PubMed] [Google Scholar]
- Harkema J. R., Plopper C. G., Hyde D. M., St George J. A., Wilson D. W., Dungworth D. L. Response of the macaque nasal epithelium to ambient levels of ozone. A morphologic and morphometric study of the transitional and respiratory epithelium. Am J Pathol. 1987 Jul;128(1):29–44. [PMC free article] [PubMed] [Google Scholar]
- Harkema J. R., Plopper C. G., Hyde D. M., Wilson D. W., St George J. A., Wong V. J. Nonolfactory surface epithelium of the nasal cavity of the bonnet monkey: a morphologic and morphometric study of the transitional and respiratory epithelium. Am J Anat. 1987 Nov;180(3):266–279. doi: 10.1002/aja.1001800308. [DOI] [PubMed] [Google Scholar]
- Haschek W. M., Morse C. C., Boyd M. R., Hakkinen P. J., Witschi H. P. Pathology of acute inhalation exposure to 3-methylfuran in the rat and hamster. Exp Mol Pathol. 1983 Dec;39(3):342–354. doi: 10.1016/0014-4800(83)90063-1. [DOI] [PubMed] [Google Scholar]
- Jiang X. Z., Buckley L. A., Morgan K. T. Pathology of toxic responses to the RD50 concentration of chlorine gas in the nasal passages of rats and mice. Toxicol Appl Pharmacol. 1983 Nov;71(2):225–236. doi: 10.1016/0041-008x(83)90339-3. [DOI] [PubMed] [Google Scholar]
- Lenz H. Three-dimensional surface representation of the cilia-free nasal mucosa of man. A scanning-electron-microscopical study. Acta Otolaryngol. 1973 Jul;76(1):47–57. doi: 10.3109/00016487309121482. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N. A., Popp J. A. Ultrastructural characterization of the nasal respiratory epithelium in the rat. Am J Anat. 1984 Jan;169(1):31–43. doi: 10.1002/aja.1001690103. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N. A., Popp J. A. Ultrastructural characterization of the nasal respiratory epithelium in the rat. Am J Anat. 1984 Jan;169(1):31–43. doi: 10.1002/aja.1001690103. [DOI] [PubMed] [Google Scholar]
- Monteiro-Riviere N. A., Popp J. A. Ultrastructural evaluation of acute nasal toxicity in the rat respiratory epithelium in response to formaldehyde gas. Fundam Appl Toxicol. 1986 Feb;6(2):251–262. [PubMed] [Google Scholar]
- Montgomery W. M., Vig P. S., Staab E. V., Matteson S. R. Computed tomography: a three-dimensional study of the nasal airway. Am J Orthod. 1979 Oct;76(4):363–375. doi: 10.1016/0002-9416(79)90223-9. [DOI] [PubMed] [Google Scholar]
- Monticello T. M., Morgan K. T., Everitt J. I., Popp J. A. Effects of formaldehyde gas on the respiratory tract of rhesus monkeys. Pathology and cell proliferation. Am J Pathol. 1989 Mar;134(3):515–527. [PMC free article] [PubMed] [Google Scholar]
- Patra A. L. Comparative anatomy of mammalian respiratory tracts: the nasopharyngeal region and the tracheobronchial region. J Toxicol Environ Health. 1986;17(2-3):163–174. doi: 10.1080/15287398609530813. [DOI] [PubMed] [Google Scholar]
- Patra A. L., Gooya A., Ménache M. G. A morphometric comparison of the nasopharyngeal airway of laboratory animals and humans. Anat Rec. 1986 May;215(1):42–50. doi: 10.1002/ar.1092150107. [DOI] [PubMed] [Google Scholar]
- Schreider J. P., Raabe O. G. Anatomy of the nasal-pharyngeal airway of experimental animals. Anat Rec. 1981 Jun;200(2):195–205. doi: 10.1002/ar.1092000208. [DOI] [PubMed] [Google Scholar]