Abstract
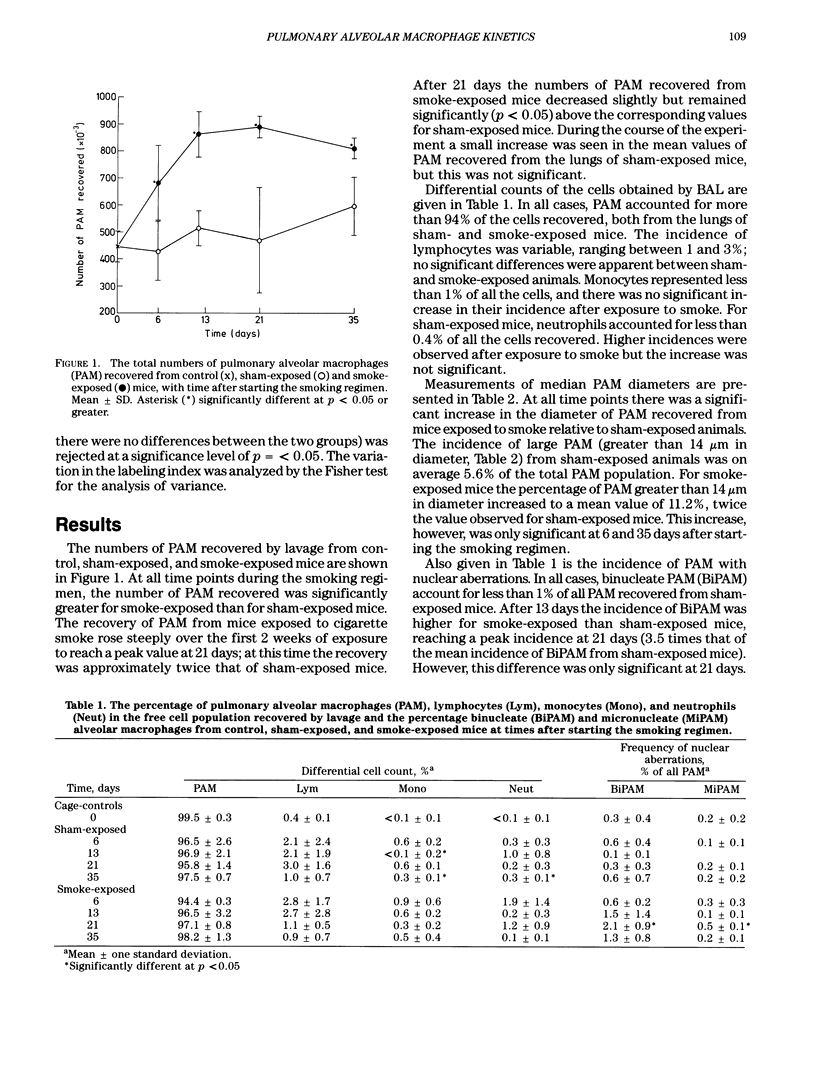
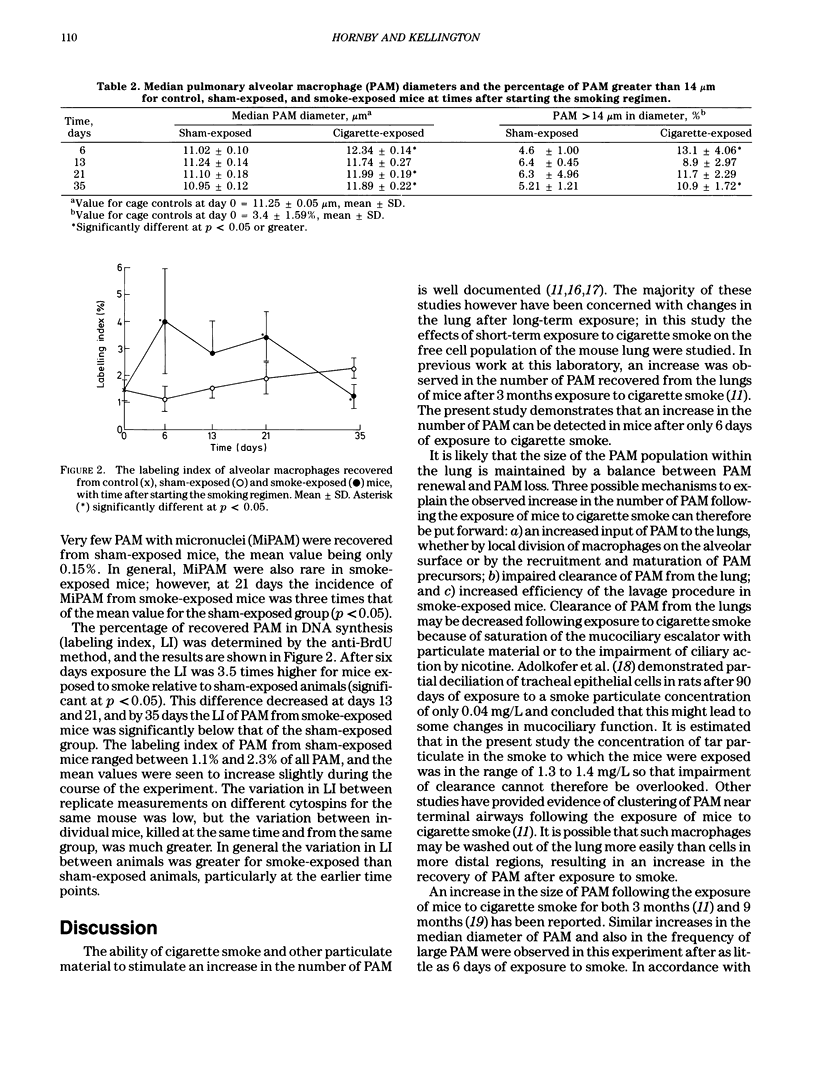
Traditional methods to determine the proportion of cells in S-phase use radiolabeled precursors of DNA, such as 3H-thymidine, which become incorporated into DNA during its synthesis and are visualized either in tissue sections or in cell preparations by autoradiography. At the Harwell Laboratory the effects of inhaled alpha-emitting actinides on the pulmonary alveolar macrophage population of the rodent lung are being studied. For this research the use of an autoradiographic technique to determine the proportion of cells in S-phase is inappropriate, because of the possible presence of competing sources of radioactivity in the cells under investigation. Consequently, an alternative method has been developed. In this method, 5-bromodeoxyuridine (BrdU), an analogue of thymidine, is incorporated into cells undergoing DNA synthesis. Fluorescein-conjugated monoclonal antibodies, highly specific for BrdU substituted DNA, are available commercially and may be used as a probe for BrdU-labeled cells. This technique for identifying cells in S-phase has been described previously for the flow cytometric analysis of cell suspensions and for cells in tissue sections. An adaptation of this technique for use on cytocentrifuge preparations of cells recovered from mouse lung by bronchoalveolar lavage has been developed and its use is described. Some preliminary results of a short-term experiment with CBA/H mice to determine the effects of exposure to cigarette smoke on the DNA synthesis of alveolar macrophages are also included.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Role of monocytes and interstitial cells in the generation of alveolar macrophages II. Kinetic studies after carbon loading. Lab Invest. 1980 May;42(5):518–524. [PubMed] [Google Scholar]
- Bitterman P. B., Saltzman L. E., Adelberg S., Ferrans V. J., Crystal R. G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984 Aug;74(2):460–469. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggle J. E., Tarling J. D. The proliferation kinetics of pulmonary alveolar macrophages. J Leukoc Biol. 1984 Mar;35(3):317–327. doi: 10.1002/jlb.35.3.317. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Roberts L. M., Keast D. DNA synthesis in cell cultures following repeated exposure to fresh cigarette smoke. Experientia. 1975 Jan 15;31(1):109–110. doi: 10.1007/BF01924706. [DOI] [PubMed] [Google Scholar]
- Matulionis D. H. Reaction of macrophages to cigarette smoke. I. Recruitment of pulmonary macrophages. Arch Environ Health. 1979 Sep-Oct;34(5):293–298. doi: 10.1080/00039896.1979.10667419. [DOI] [PubMed] [Google Scholar]
- Matulionis D. H. Reaction of macrophages to cigarette smoke. II. Immigration of macrophages to the lungs. Arch Environ Health. 1979 Sep-Oct;34(5):298–301. doi: 10.1080/00039896.1979.10667420. [DOI] [PubMed] [Google Scholar]
- Matulionis D. H., Simmerman L. A. Chronic cigarette smoke inhalation and aging in mice: 2. Quantitation of the pulmonary macrophage response. Exp Lung Res. 1985;9(3-4):309–326. doi: 10.3109/01902148509057530. [DOI] [PubMed] [Google Scholar]
- Matulionis D. H., Traurig H. H. In situ response of lung macrophages and hydrolase activities to cigarette smoke. Lab Invest. 1977 Sep;37(3):314–326. [PubMed] [Google Scholar]
- Moores S. R., Talbot R. J., Evans N., Lambert B. E. Macrophage depletion of mouse lung following inhalation of 239PuO2. Radiat Res. 1986 Mar;105(3):387–404. [PubMed] [Google Scholar]
- Rasmussen R. E., Boyd C. H., Dansie D. R., Kouri R. E., Henry C. J. DNA replication and unscheduled DNA synthesis in lungs of mice exposed to cigarette smoke. Cancer Res. 1981 Jul;41(7):2583–2588. [PubMed] [Google Scholar]
- Rylander R. Free lung cell studies in cigarette smoke inhalation experiments. Scand J Respir Dis. 1971;52(2):121–128. [PubMed] [Google Scholar]
- Sawyer R. T. The cytokinetic behavior of pulmonary alveolar macrophages in monocytopenic mice. J Leukoc Biol. 1986 Jan;39(1):89–99. doi: 10.1002/jlb.39.1.89. [DOI] [PubMed] [Google Scholar]
- Schmid W. The micronucleus test. Mutat Res. 1975 Feb;31(1):9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- Shellito J., Esparza C., Armstrong C. Maintenance of the normal rat alveolar macrophage cell population. The roles of monocyte influx and alveolar macrophage proliferation in situ. Am Rev Respir Dis. 1987 Jan;135(1):78–82. doi: 10.1164/arrd.1987.135.1.78. [DOI] [PubMed] [Google Scholar]
- Speit G., Vogel W. Detection of bromodeoxyuridine incorporation in mammalian chromosomes by a bromodeoxyuridine antibody. II. Demonstration of sister chromatid exchanges. Chromosoma. 1986;94(2):103–106. doi: 10.1007/BF00286987. [DOI] [PubMed] [Google Scholar]
- Springmeyer S. C., Altman L. C., Kopecky K. J., Deeg H. J., Storb R. Alveolar macrophage kinetics and function after interruption of canine marrow function. Am Rev Respir Dis. 1982 Mar;125(3):347–351. doi: 10.1164/arrd.1982.125.3.347. [DOI] [PubMed] [Google Scholar]
- Spurzem J. R., Saltini C., Rom W., Winchester R. J., Crystal R. G. Mechanisms of macrophage accumulation in the lungs of asbestos-exposed subjects. Am Rev Respir Dis. 1987 Aug;136(2):276–280. doi: 10.1164/ajrccm/136.2.276. [DOI] [PubMed] [Google Scholar]
- Talbot R. J., Morgan A., Moores S. R., Matulionis D. H. Preliminary studies of the interaction between 239PuO2 and cigarette smoke in the mouse lung. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Jun;51(6):1101–1110. doi: 10.1080/09553008714551391. [DOI] [PubMed] [Google Scholar]
- Wright E. S., White D. M., Brady A. N., Li L. C., D'Arcy J. B., Smiler K. L. DNA synthesis in pulmonary alveolar macrophages and type II cells: effects of ozone exposure and treatment with alpha-difluoromethylornithine. J Toxicol Environ Health. 1987;21(1-2):15–26. doi: 10.1080/15287398709530999. [DOI] [PubMed] [Google Scholar]
- van Furth R., Blussé van Oud Alblas A. The current view on the origin of pulmonary macrophages. Pathol Res Pract. 1982 Oct;175(1):38–49. doi: 10.1016/S0344-0338(82)80041-1. [DOI] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]


