Abstract
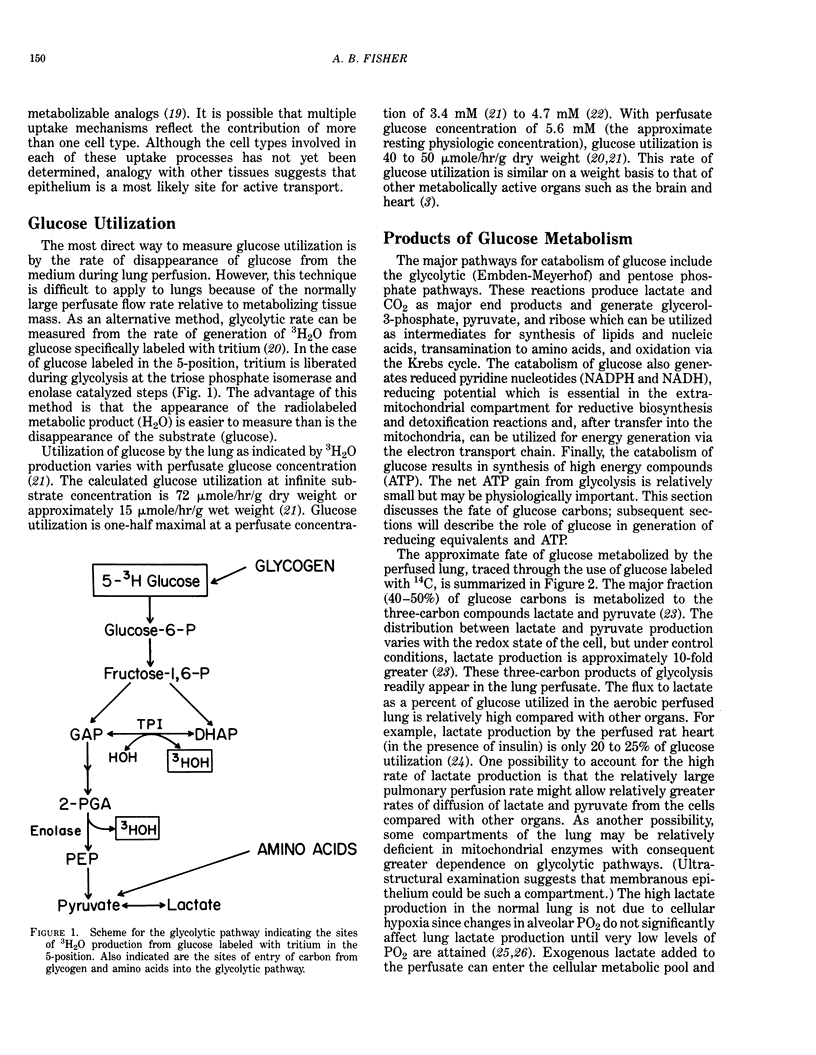
The lung is a metabolically active organ that is engaged in secretion, clearance and other maintenance functions that require reducing potential, energy and substrates for biosynthesis. These metabolic requirements are met in part through uptake and catabolism of glucose which represents the major fuel utilized by lung tissues. Gluconeogenesis does not occur, and glycogen stores are limited so that the lung depends on the circulation for its glucose requirement. Other substrates can be metabolized by lung and contribute to the metabolic pool although their role has been less thoroughly studied. Glucose is catabolized in the lung by cytoplasmic and mitochondrial pathways that are responsive to regulatory mechanisms as in other tissues. Activity of the pentose cycle pathway of glucose catabolism is relatively high and generates the NADPH required for biosynthesis of lipid, detoxification reactions, and protection against oxidant stress. The ATP content of the lung is maintained by oxidative metabolism at levels comparable to other metabolically active organs. Alterations in lung intermediary metabolism may depress amine clearance, alter lung permeability, and influence the lung response to oxidant stress.
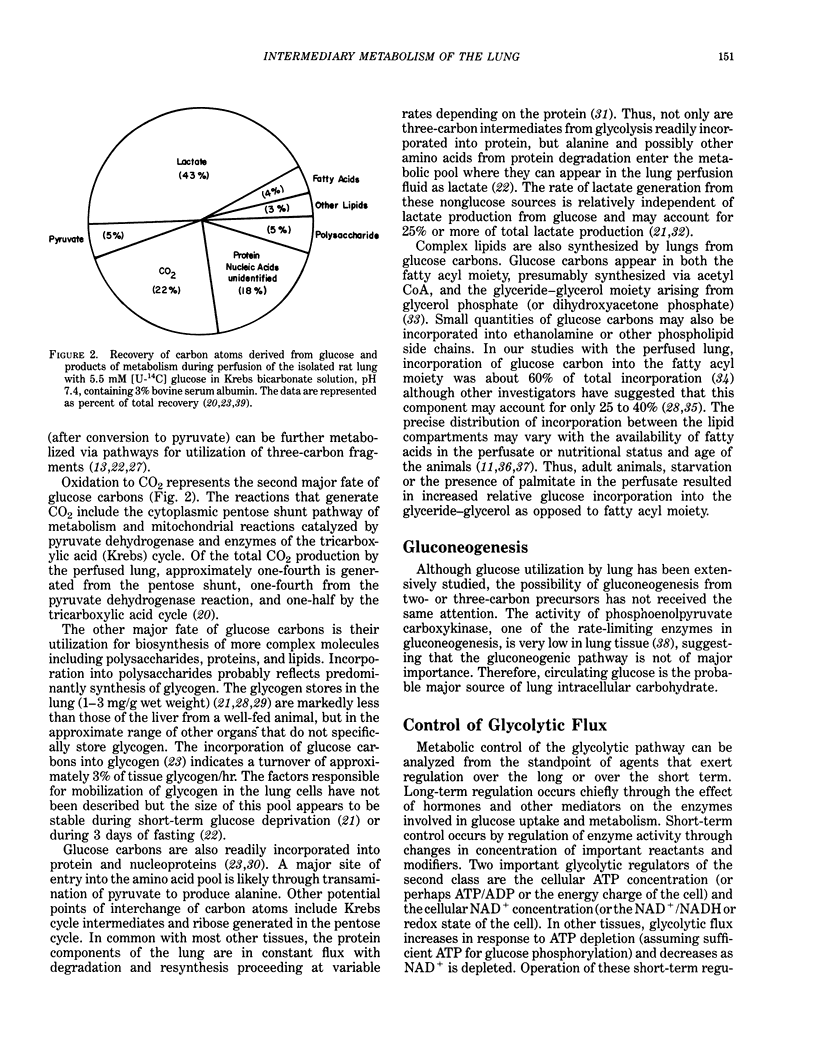
Full text
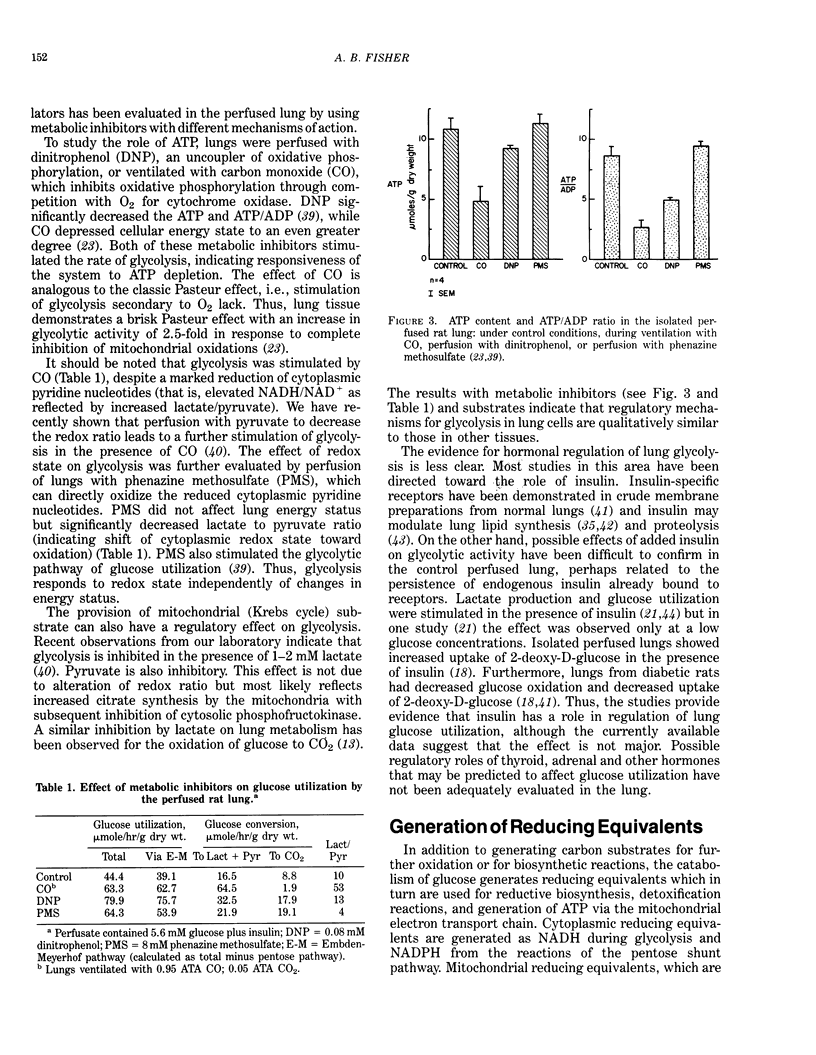
PDF

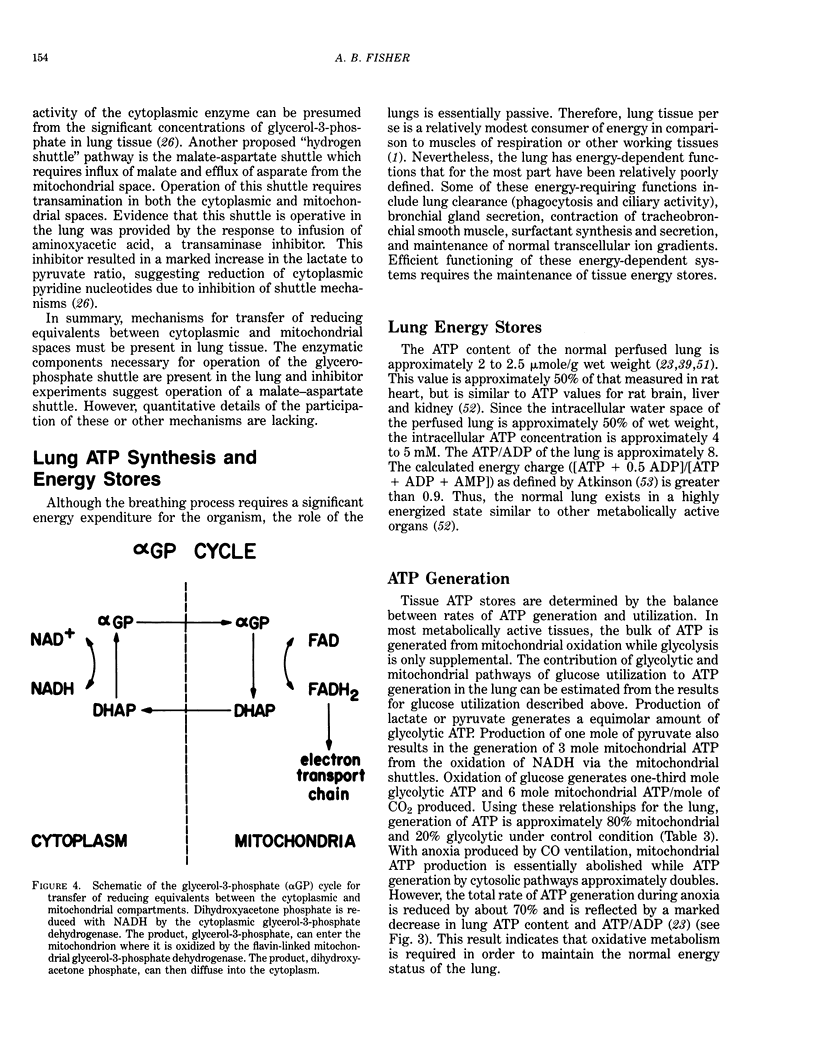




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E., Walton G. M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967 Jul 10;242(13):3239–3241. [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Alterations of glucose metabolism during perfusion of rat lung with paraquat. Am J Physiol. 1978 Jun;234(6):E653–E659. doi: 10.1152/ajpendo.1978.234.6.E653. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Glucose metabolism in rat lung during exposure to hyperbaric O2. J Appl Physiol Respir Environ Exerc Physiol. 1979 May;46(5):943–949. doi: 10.1152/jappl.1979.46.5.943. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Metabolic response to carbon monoxide by isolated rat lungs. Am J Physiol. 1976 Mar;230(3):658–663. doi: 10.1152/ajplegacy.1976.230.3.658. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Pentose cycle activity of the isolated perfused rat lung. Am J Physiol. 1976 Nov;231(5 Pt 1):1527–1532. doi: 10.1152/ajplegacy.1976.231.5.1527. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B., Rabinowitz J. L. Effect of hypoxia on incorporation of glucose carbons into lipids by isolated rat lung. Am J Physiol. 1974 Nov;227(5):1103–1108. doi: 10.1152/ajplegacy.1974.227.5.1103. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Fisher A. B. Stimulation of rat lung metabolism with 2,4-dinitrophenol and phenazine methosulfate. Am J Physiol. 1976 Sep;231(3):898–902. doi: 10.1152/ajplegacy.1976.231.3.898. [DOI] [PubMed] [Google Scholar]
- Bassett D. J., Hamosh M., Hamosh P., Rabinowitz J. L. Pathways of palmitate metabolism in the isolated rat lung. Exp Lung Res. 1981 Mar;2(1):37–47. doi: 10.3109/01902148109052301. [DOI] [PubMed] [Google Scholar]
- Buechler K. F., Rhoades R. A. Fatty acid synthesis in the perfused rat lung. Biochim Biophys Acta. 1980 Aug 11;619(2):186–195. doi: 10.1016/0005-2760(80)90067-3. [DOI] [PubMed] [Google Scholar]
- Bus J. S., Aust S. D., Gibson J. E. Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem Biophys Res Commun. 1974 Jun 4;58(3):749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- Chance B. Reaction of oxygen with the respiratory chain in cells and tissues. J Gen Physiol. 1965 Sep;49(1 Suppl):163–195. doi: 10.1085/jgp.49.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M. J., Whitney P., Jr, Massaro D. Protein metabolism in lung: use of isolated perfused lung to study protein degradation. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jul;47(1):72–78. doi: 10.1152/jappl.1979.47.1.72. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- Devereux T. R., Serabjit-Singh C. J., Slaughter S. R., Wolf C. R., Philpot R. M., Fouts J. R. Identification of cytochrome P-450 isozymes in nonciliated bronchiolar epithelial (Clara) and alveolar type II cells isolated from rabbit lung. Exp Lung Res. 1981 Aug;2(3):221–230. doi: 10.3109/01902148109052317. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Block E. R., Pietra G. Environmental influences on uptake of serotonin and other amines. Environ Health Perspect. 1980 Apr;35:191–198. doi: 10.1289/ehp.8035191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. B., Dodia C., Linask J. Perfusate composition and edema formation in isolated rat lungs. Exp Lung Res. 1980 Mar;1(1):13–21. doi: 10.3109/01902148009057509. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Dodia C. Lung as a model for evaluation of critical intracellular PO2 and PCO. Am J Physiol. 1981 Jul;241(1):E47–E50. doi: 10.1152/ajpendo.1981.241.1.E47. [DOI] [PubMed] [Google Scholar]
- Fisher A. B. Energy status of the rat lung after exposure to elevated PO2. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):56–59. doi: 10.1152/jappl.1978.45.1.56. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Furia L., Berman H. Metabolism of rat granular pneumocytes isolated in primary culture. J Appl Physiol Respir Environ Exerc Physiol. 1980 Oct;49(4):743–750. doi: 10.1152/jappl.1980.49.4.743. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Furia L., Chance B. Evaluation of redox state of isolated perfused rat lung. Am J Physiol. 1976 May;230(5):1198–1204. doi: 10.1152/ajplegacy.1976.230.5.1198. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Huber G. A., Bassett D. J. Oxidation of alpha-glycerophosphate by mitochondria from lungs of rabbits, sheep and pigeons. Comp Biochem Physiol B. 1975 Jan 15;50(1):5–8. doi: 10.1016/0305-0491(75)90289-8. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Huber G. A., Furia L., Bassett D., Rabinowitz J. L. Evidence for lipid synthesis by the dihydroxyacetone phosphate pathway in rabbit lung subcellular fractions. J Lab Clin Med. 1976 Jun;87(6):1033–1040. [PubMed] [Google Scholar]
- Fisher A. B., Hyde R. W., Reif J. S. Insensitivity of the alveolar septum to local hypoxia. Am J Physiol. 1972 Oct;223(4):770–776. doi: 10.1152/ajplegacy.1972.223.4.770. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Itakura N., Dodia C., Thurman R. G. Pulmonary mixed-function oxidation: stimulation by glucose and the effects of metabolic inhibitors. Biochem Pharmacol. 1981 Feb 15;30(4):379–383. doi: 10.1016/0006-2952(81)90070-8. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Scarpa A., LaNoue K. F., Bassett D., Williamson J. R. Respiration of rat lung mitochondria and the influence of Ca 2+ on substrate utilization. Biochemistry. 1973 Mar 27;12(7):1438–1445. doi: 10.1021/bi00731a026. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Steinberg H., Bassett D. Energy utilization by the lung. Am J Med. 1974 Sep;57(3):437–446. doi: 10.1016/0002-9343(74)90137-5. [DOI] [PubMed] [Google Scholar]
- Fisher A. B., Steinberg H., Dodia C. Reversal of 2-deoxyglucose inhibition of serotonin uptake in isolated guinea pig lung. J Appl Physiol Respir Environ Exerc Physiol. 1979 Mar;46(3):447–450. doi: 10.1152/jappl.1979.46.3.447. [DOI] [PubMed] [Google Scholar]
- Fisher H. K., Clements J. A., Wright R. R. Enhancement of oxygen toxicity by the herbicide paraquat. Am Rev Respir Dis. 1973 Feb;107(2):246–252. doi: 10.1164/arrd.1973.107.2.246. [DOI] [PubMed] [Google Scholar]
- Fricke R. F., Longmore W. J. Effects of insulin and diabetes on 2-deoxy-D-glucose uptake by the isolated perfused rat lung. J Biol Chem. 1979 Jun 25;254(12):5092–5098. [PubMed] [Google Scholar]
- Habliston D. L., Whitaker C., Hart M. A., Ryan U. S., Ryan J. W. Isolation and culture of endothelial cells from the lungs of small animals. Am Rev Respir Dis. 1979 Jun;119(6):853–868. doi: 10.1164/arrd.1979.119.6.853. [DOI] [PubMed] [Google Scholar]
- Hamosh M., Shechter Y., Hamosh P. Metabolic activity of developing rabbit lung. Pediatr Res. 1978 Feb;12(2):95–100. doi: 10.1203/00006450-197802000-00006. [DOI] [PubMed] [Google Scholar]
- Junod A. F. Uptake, metabolism and efflux of 14 C-5-hydroxytryptamine in isolated perfused rat lungs. J Pharmacol Exp Ther. 1972 Nov;183(2):341–355. [PubMed] [Google Scholar]
- KATZ J., WOOD H. G. The use of C14O2 yields from glucose-1- and -6-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem. 1963 Feb;238:517–523. [PubMed] [Google Scholar]
- Kerr J. S., Baker N. J., Bassett D. J., Fisher A. B. Effect of perfusate glucose concentration on rat lung glycolysis. Am J Physiol. 1979 Mar;236(3):E229–E233. doi: 10.1152/ajpendo.1979.236.3.E229. [DOI] [PubMed] [Google Scholar]
- Kerr J. S., Fisher A. B., Kleinzeller A. Transport of glucose analogues in rat lung. Am J Physiol. 1981 Sep;241(3):E191–E195. doi: 10.1152/ajpendo.1981.241.3.E191. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y., Yoneda K. The type II epithelial cell of the lung. I. Method of isolation. Lab Invest. 1974 Jan;30(1):76–84. [PubMed] [Google Scholar]
- LANDAU B. R., BERNSTEIN L., WILSON T. H. Hexose transport by hamster intestine in vitro. Am J Physiol. 1962 Aug;203:237–240. doi: 10.1152/ajplegacy.1962.203.2.237. [DOI] [PubMed] [Google Scholar]
- Longmore W. J., Mourning J. T. Lactate production in isolated perfused rat lung. Am J Physiol. 1976 Aug;231(2):351–354. doi: 10.1152/ajplegacy.1976.231.2.351. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C., Greenleaf R. D., Clements J. A. Isolation and properties of type II alveolar cells from rat lung. Am Rev Respir Dis. 1977 Jun;115(6):1015–1026. doi: 10.1164/arrd.1977.115.6.1015. [DOI] [PubMed] [Google Scholar]
- Mastafa M. G., Cross C. E. Lung cell mitochondria: rapid oxidation of glycerol-l-phosphate but slow oxidation of 3-hydroxybutyrate. Am Rev Respir Dis. 1974 Feb;109(2):301–303. doi: 10.1164/arrd.1974.109.2.301. [DOI] [PubMed] [Google Scholar]
- Morishige W. K., Uetake C. A., Greenwood F. C., Akaka J. Pulmonary insulin responsivitiy: in vivo effects of insulin on the diabetic rat lung and specific insulin binding to lung receptors in normal rats. Endocrinology. 1977 Jun;100(6):1710–1722. doi: 10.1210/endo-100-6-1710. [DOI] [PubMed] [Google Scholar]
- Moxley M. A., Longmore W. J. Effect of experimental diabetes and insulin on lipid metabolism in the isolated perfused rat lung. Biochim Biophys Acta. 1977 Aug 24;488(2):218–224. doi: 10.1016/0005-2760(77)90179-5. [DOI] [PubMed] [Google Scholar]
- Picciano P., Rosenbaum R. M. The type 1 alveolar lining cells of the mammalian lung. I. Isolation and enrichment from dissociated adult rabbit lung. Am J Pathol. 1978 Jan;90(1):99–122. [PMC free article] [PubMed] [Google Scholar]
- Reiss O. K. Studies of lung metabolism. I. Isolation and properties of subcellular fractions from rabbit lung. J Cell Biol. 1966 Jul;30(1):45–57. doi: 10.1083/jcb.30.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades R. A. Influence of starvation on the lung: effect on glucose and palmitate utilization. J Appl Physiol. 1975 Mar;38(3):513–516. doi: 10.1152/jappl.1975.38.3.513. [DOI] [PubMed] [Google Scholar]
- Rhoades R. A. Net uptake of glucose, glycerol, and fatty acids by the isolated perfused rat lung. Am J Physiol. 1974 Jan;226(1):144–149. doi: 10.1152/ajplegacy.1974.226.1.144. [DOI] [PubMed] [Google Scholar]
- Rhoades R. A., Shaw M. E., Eskew M. L., Wali S. Lactate metabolism in perfused rat lung. Am J Physiol. 1978 Dec;235(6):E619–E623. doi: 10.1152/ajpendo.1978.235.6.E619. [DOI] [PubMed] [Google Scholar]
- Scholz R. W., Evans R. M. Pulmonary fatty acid synthesis. II. Amino acids as fatty acid precursors in rat lung. Am J Physiol. 1977 Apr;232(4):E364–E369. doi: 10.1152/ajpendo.1977.232.4.E364. [DOI] [PubMed] [Google Scholar]
- Scholz R. W. Lipid metabolism by rat lung in vitro. Utilization of citrate by normal and starved rats. Biochem J. 1972 Mar;126(5):1219–1224. doi: 10.1042/bj1261219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R. W., Rhoades R. A. Lipid metabolism by rat lung in vitro. Effect of starvation and re-feeding on utilization of (U- 14 C)glucose by lung slices. Biochem J. 1971 Sep;124(2):257–264. doi: 10.1042/bj1240257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. E., Rhoades R. A. Substrate metabolism in the perfused lung: response to changes in circulating glucose and palmitate levels. Lipids. 1977 Nov;12(11):930–935. doi: 10.1007/BF02533313. [DOI] [PubMed] [Google Scholar]
- Spear R. K., Lumeng L. A method for isolating lung mitochondria from rabbits, rats, and mice with improved respiratory characteristics. Anal Biochem. 1978 Oct 1;90(1):211–219. doi: 10.1016/0003-2697(78)90025-8. [DOI] [PubMed] [Google Scholar]
- Steinberg H., Bassett D. J., Fisher A. B. Depression of pulmonary 5-hydroxytryptamine uptake by metabolic inhibitors. Am J Physiol. 1975 May;228(5):1298–1303. doi: 10.1152/ajplegacy.1975.228.5.1298. [DOI] [PubMed] [Google Scholar]
- Stubbs W. A., Morgan I., Lloyd B., Alberti K. G. The effect of insulin on lung metabolism in the rat. Clin Endocrinol (Oxf) 1977 Sep;7(3):181–184. doi: 10.1111/j.1365-2265.1977.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Thet L. A., Delaney M. D., Gregorio C. A., Massaro D. Protein metabolism by rat lung: influence of fasting, glucose, and insulin. J Appl Physiol Respir Environ Exerc Physiol. 1977 Sep;43(3):463–467. doi: 10.1152/jappl.1977.43.3.463. [DOI] [PubMed] [Google Scholar]
- Tierney D., Ayers L., Herzog S., Yang J. Pentose pathway and production of reduced nicotinamide adenine dinucleotide phosphate. A mechanism that may protect lungs from oxidants. Am Rev Respir Dis. 1973 Dec;108(6):1348–1351. doi: 10.1164/arrd.1973.108.6.1348. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J. Sugar, amino acid, and Na+ cotransport in the proximal tubule. Annu Rev Physiol. 1979;41:181–195. doi: 10.1146/annurev.ph.41.030179.001145. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- Wallace H. W., Stein T. P., Liquori E. M. Lactate and lung metabolism. J Thorac Cardiovasc Surg. 1974 Nov;68(5):810–814. [PubMed] [Google Scholar]
- Wolfe R. R., Hochachka P. W., Trelstad R. L., Burke J. F. Lactate oxidation in perfused rat lung. Am J Physiol. 1979 Mar;236(3):E276–E282. doi: 10.1152/ajpendo.1979.236.3.E276. [DOI] [PubMed] [Google Scholar]
- Yeager H., Jr, Massaro D. Glucose metabolism and glycoprotein synthesis by lung slices. J Appl Physiol. 1972 Apr;32(4):477–482. doi: 10.1152/jappl.1972.32.4.477. [DOI] [PubMed] [Google Scholar]
- Young S. L., O'Neil J. J., Kasuyama R. S., Tierney D. F. Glucose utilization by edematous rat lungs. Lung. 1980;157(3):165–177. doi: 10.1007/BF02713613. [DOI] [PubMed] [Google Scholar]
- Young S. L., O'Neil J. J., Kasuyama R. S., Tierney D. F. Glucose utilization by edematous rat lungs. Lung. 1980;157(3):165–177. doi: 10.1007/BF02713613. [DOI] [PubMed] [Google Scholar]


