Abstract
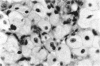
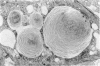
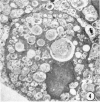
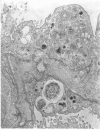
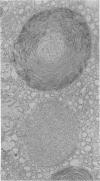
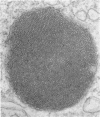
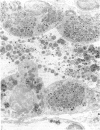
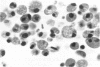
Administration of amphiphilic drugs to experimental animals causes formation of myelinoid bodies in many cell types, accumulation of foamy macrophages in pulmonary alveoli and pulmonary alveolar proteinosis. These changes are the result of an interaction between the drugs and phospholipids which leads to an alteration in physicochemical properties of the phospholipids. Impairment of the digestion of altered pulmonary secretions in phagosomes of macrophages results in accumulation of foam cells in pulmonary alveoli. Impairment of the metabolism of altered phospholipids removed by autophagy induces an accumulation of myelinoid bodies. The administration of amphiphilic compounds thus causes pulmonary intra-alveolar histiocytosis which is a part of a drug-induced lysosomal storage or generalized lipidosis. The accumulation of drug-lipid complexes in myelinoid bodies and in pulmonary foam cells may lead to alteration of cellular functioning and to clinical disease. Currently over 50 amphiphilic drugs are known. Unique pharmacological properties necessitate clinical use of some of these drugs. The occurrence and severity of potential clinical side effects depend on the nature of each drug, dosage and duration of treatment, simultaneous administration of other drugs and foods, individual metabolic pattern of the patient and other factors. Further studies on factors preventing and potentiating adverse effects of amphiphilic drugs are indicated.
Full text
PDF

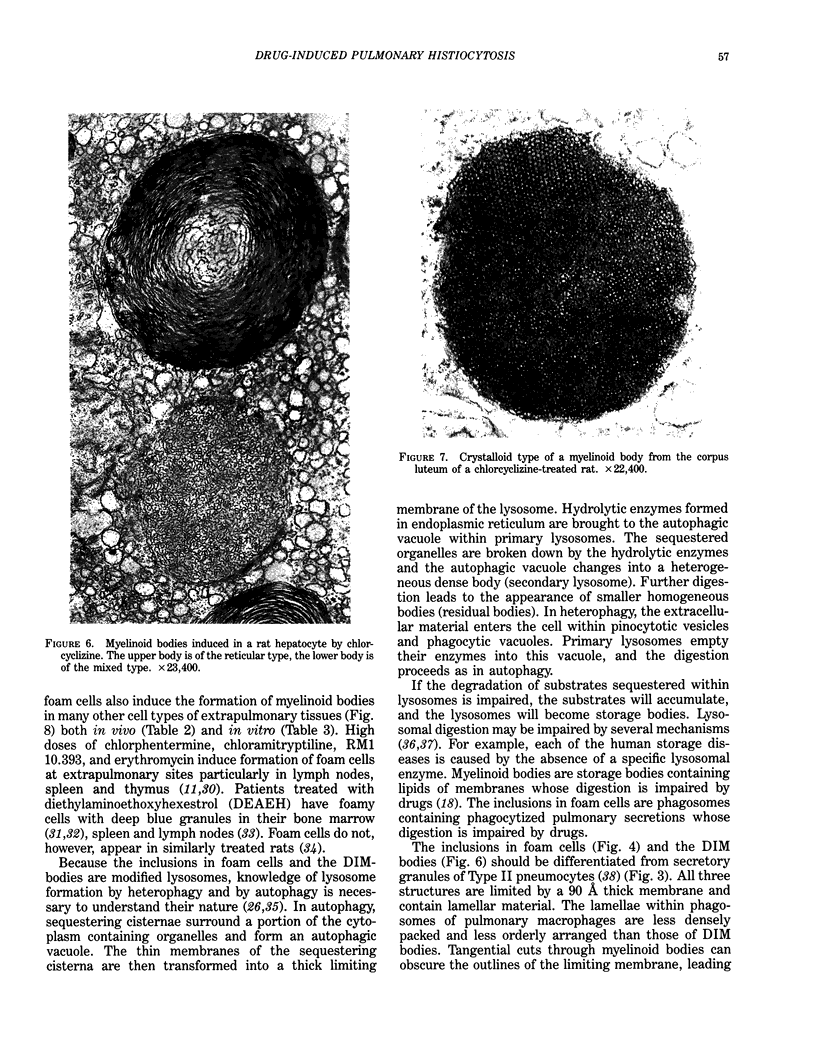
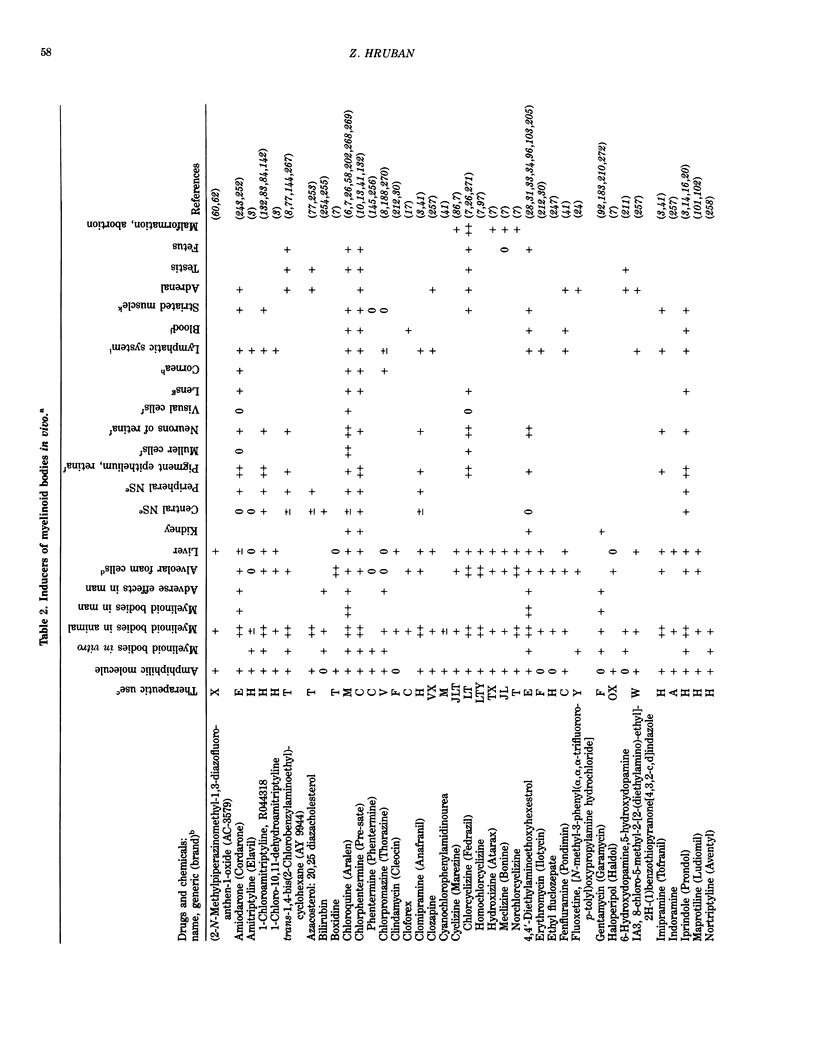
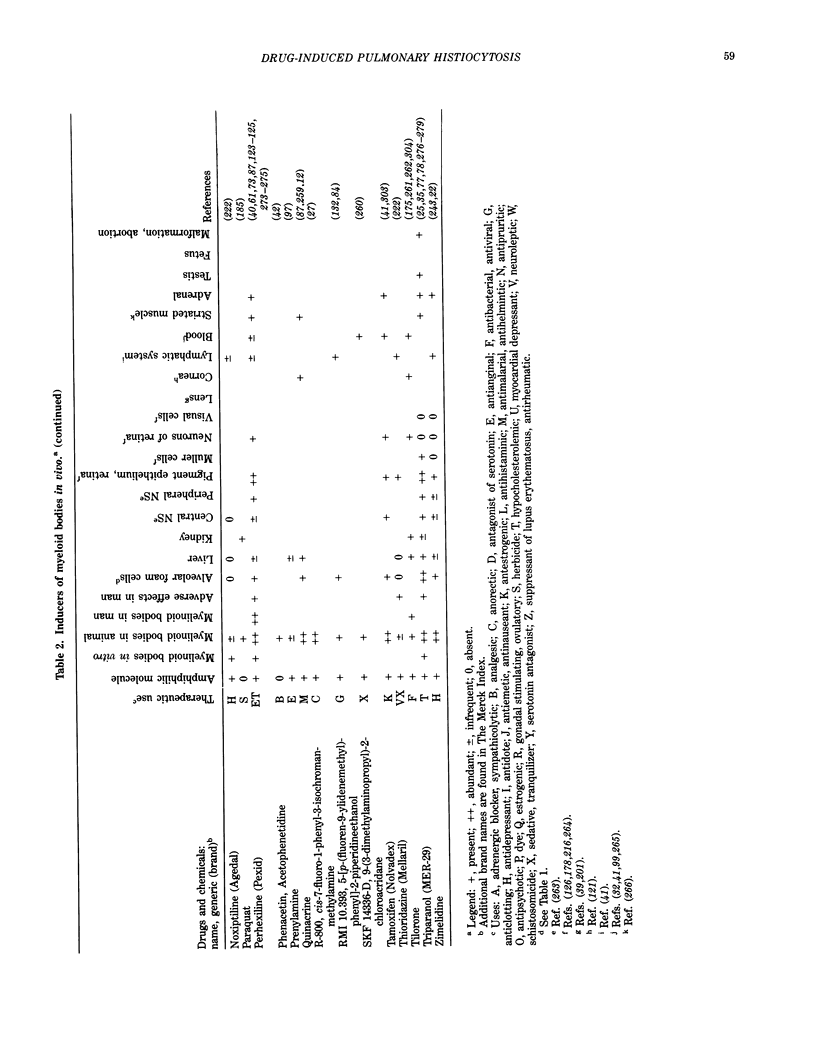

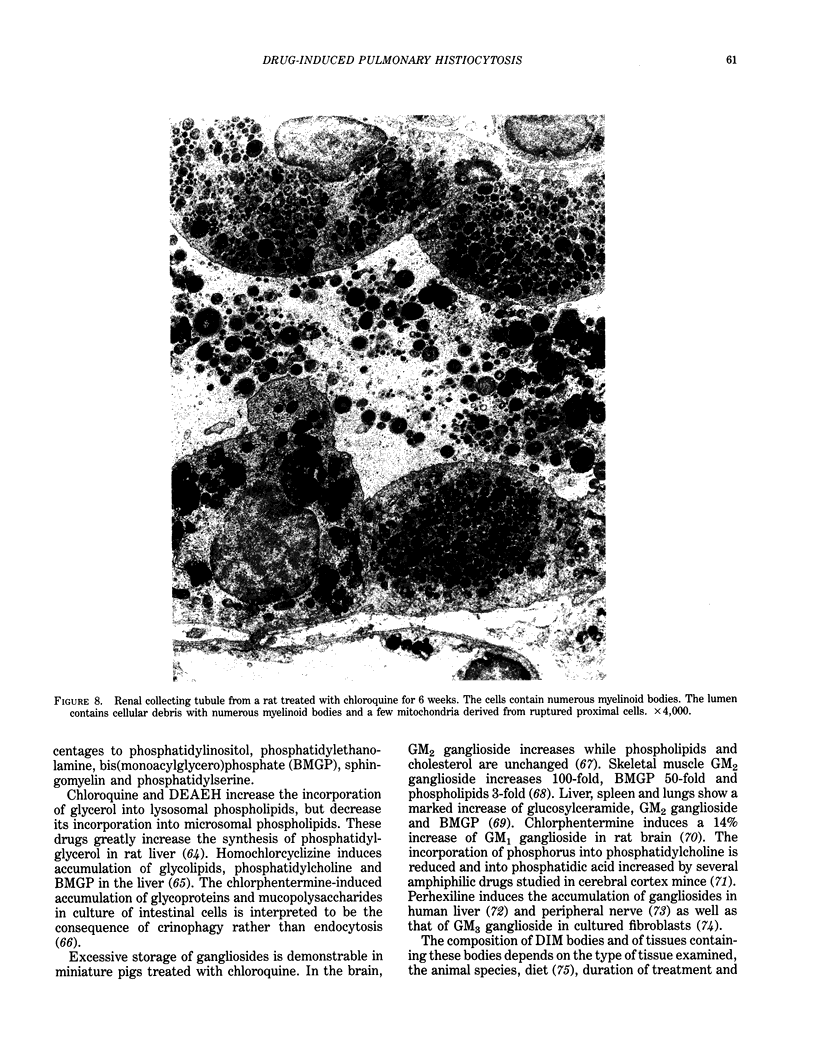














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R., Hendy R. J. Irreversible lysosomal damage induced by chloroquine in the retinae of pigmented and albino rats. Exp Mol Pathol. 1970 Apr;12(2):185–200. doi: 10.1016/0014-4800(70)90049-3. [DOI] [PubMed] [Google Scholar]
- Abraham R., Hendy R. Effects of chronic chloroquine treatment on lysosomes of rat liver cells. Exp Mol Pathol. 1970 Apr;12(2):148–159. doi: 10.1016/0014-4800(70)90046-8. [DOI] [PubMed] [Google Scholar]
- Abraham R., Hendy R., Grasso P. Formation of myeloid bodies in rat liver lysosomes after chloroquine administration. Exp Mol Pathol. 1968 Oct;9(2):212–229. doi: 10.1016/0014-4800(68)90037-3. [DOI] [PubMed] [Google Scholar]
- Albert C., Lüllmann-Rauch R. Ultrastructural alterations in peripheral nerve trunks of rats subchronically treated with chlorphentermine or perhexiline. Arzneimittelforschung. 1983;33(1):125–127. [PubMed] [Google Scholar]
- Alexander N. J. Ultrastructural changes in rat epididymis after vasectomy. Z Zellforsch Mikrosk Anat. 1973;136(2):177–182. doi: 10.1007/BF00307438. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Enhanced synthesis de novo of phosphatidylinositol in lymphocytes treated with cationic amphiphilic drugs. Biochem J. 1975 Jun;148(3):471–478. doi: 10.1042/bj1480471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. W., Orton T. C., Pickett R. D., Eling T. E. Accumulation of amines in the isolated perfused rabbit lung. J Pharmacol Exp Ther. 1974 May;189(2):456–466. [PubMed] [Google Scholar]
- Angevine L. S., Mehendale H. M. Chlorphentermine uptake by isolated perfused rabbit lung. Toxicol Appl Pharmacol. 1980 Feb;52(2):336–346. doi: 10.1016/0041-008x(80)90120-9. [DOI] [PubMed] [Google Scholar]
- Angevine L. S., Mehendale H. M. Effect of chlorphentermine on the pulmonary disposition of 5-hydroxytryptamine in the isolated perfused rabbit lung. Am Rev Respir Dis. 1980 Dec;122(6):891–898. doi: 10.1164/arrd.1980.122.6.891. [DOI] [PubMed] [Google Scholar]
- Angevine L. S., Ohmiya Y., Mehendale H. M. Effect of chlorphentermine pretreatment on chlorphentermine uptake by isolated perfused rat lung. Drug Metab Dispos. 1982 Jan-Feb;10(1):68–73. [PubMed] [Google Scholar]
- Ashford A., Ross J. W. Toxicity of depressant and antidepressant drugs in hyperthyroid mice. Br Med J. 1968 Apr 27;2(5599):217–218. doi: 10.1136/bmj.2.5599.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert-Tulkens G., Van Hoof F., Tulkens P. Gentamicin-induced lysosomal phospholipidosis in cultured rat fibroblasts. Quantitative ultrastructural and biochemical study. Lab Invest. 1979 Apr;40(4):481–491. [PubMed] [Google Scholar]
- Barnhart J. W. The effect of chlorcyclizine on cholesterol metabolism in mice. Toxicol Appl Pharmacol. 1974 Feb;27(2):449–455. doi: 10.1016/0041-008x(74)90216-6. [DOI] [PubMed] [Google Scholar]
- Batty H. K., Millhouse O. E. Ultrastructure of the Gunn rat substantia nigra I. Cytoplasmic changes. Acta Neuropathol. 1976 Jun 15;35(2):93–107. doi: 10.1007/BF00690556. [DOI] [PubMed] [Google Scholar]
- Beaugrand M., Poupon R., Lévy V. G., Callard P., Lageron A., Lecomte D., Darnis F., Ferrier J. P. Lésions hépatiques dues au maléate de perhexiline. Etude clinique, biologique, histopathologique, ultrastructurale et biochimique de 7 cas. Gastroenterol Clin Biol. 1978;2(6-7):579–588. [PubMed] [Google Scholar]
- Bermejo J., Fernandez P., Tato F., Belmonte A. Desmethylimipramine induced changes in liposomal membrances. Res Commun Chem Pathol Pharmacol. 1974 May;8(1):101–113. [PubMed] [Google Scholar]
- Bhagwat A. G., Wentworth P., Conen P. E. Observations on the relationship of desquamative interstitial pneumonia and pulmonary alveolar proteinosis in childhood: a pathologic and experimental study. Chest. 1970 Oct;58(4):326–332. doi: 10.1378/chest.58.4.326. [DOI] [PubMed] [Google Scholar]
- Bickel M. H., Minder R. Metabolism and biliary excretion of the lipophilic drug molecules, imipramine and desmethylimipramine in the rat. II. Uptake into bile micelles. Biochem Pharmacol. 1970 Aug;19(8):2437–2443. doi: 10.1016/0006-2952(70)90268-6. [DOI] [PubMed] [Google Scholar]
- Bingham E., Barkley W., Zerwas M., Stemmer K., Taylor P. Responses of alveolar macrophages to metals. I. Inhalation of lead and nickel. Arch Environ Health. 1972 Dec;25(6):406–414. doi: 10.1080/00039896.1972.10666195. [DOI] [PubMed] [Google Scholar]
- Blanchette-Mackie E. J., Scow R. O. Retention of lipolytic products in chylomicrons incubated with lipoprotein lipase: electron microscope study. J Lipid Res. 1976 Jan;17(1):57–67. [PubMed] [Google Scholar]
- Bleistein J., Debuch H., Gunawan J. Metabolism of phosphatidylglycerol by liver lysosomes of chloroquine-pretreated rats. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1445–1448. [PubMed] [Google Scholar]
- Blohm T. R. Drug-induced lysosomal lipidosis: biochemical interpretations. Pharmacol Rev. 1978 Dec;30(4):593–603. [PubMed] [Google Scholar]
- Blok J., Mulder-Stapel A. A., Ginsel L. A., Daems W. T. The effect of chloroquine on lysosomal function and cell-coat glycoprotein transport in the absorptive cells of cultured human small-intestinal tissue. Cell Tissue Res. 1981;218(2):227–251. doi: 10.1007/BF00210340. [DOI] [PubMed] [Google Scholar]
- Bockhardt H., Lüllmann-Rauch R. Zimelidine-induced lipidosis in rats. Acta Pharmacol Toxicol (Copenh) 1980 Jul;47(1):45–48. doi: 10.1111/j.1600-0773.1980.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Bowley M., Cooling J., Burditt S. L., Brindley D. N. The effects of amphiphilic cationic drugs and inorganic cations on the activity of phosphatidate phosphohydrolase. Biochem J. 1977 Sep 1;165(3):447–454. doi: 10.1042/bj1650447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer D. B., Heath D., Asquith P. Electron microscopy of desquamative interstitial pneumonia. J Pathol. 1969 Feb;97(2):317–323. doi: 10.1002/path.1710970217. [DOI] [PubMed] [Google Scholar]
- Brindley D. N., Allan D., Michell R. H. Letter: The redirection of glyceride and phospholipid synthesis by drugs including chlorpromazine, fenfluramine, imipramine, mepyramine and local anaesthetics. J Pharm Pharmacol. 1975 Jun;27(6):462–464. [PubMed] [Google Scholar]
- Brindley D. N., Bowley M. Drugs affecting the synthesis of glycerides and phospholipids in rat liver. The effects of clofibrate, halofenate, fenfluramine, amphetamine, cinchocaine, chlorpromazine, demethylimipramine, mepyramine and some of their derivatives. Biochem J. 1975 Jun;148(3):461–469. doi: 10.1042/bj1480461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Clay M. F., Collins M. M., Eden A. C., McDermott M. Effects of chlorphentermine hydrochloride on the surface tension properties of the rat lung. J Physiol. 1975 Feb;245(2):105P–106P. [PubMed] [Google Scholar]
- Brosnan C. F., Bunge M. B., Murray M. R. The response of lysosomes in cultured neurons to chlorpromazine. J Neuropathol Exp Neurol. 1970 Jul;29(3):337–353. doi: 10.1097/00005072-197007000-00001. [DOI] [PubMed] [Google Scholar]
- Brown E. A. THe localization, metabolism and effects of drugs and toxicants in lung. Drug Metab Rev. 1974;3(1):33–87. doi: 10.3109/03602537408993738. [DOI] [PubMed] [Google Scholar]
- Brunk U., Ericsson J. L., Pontén J., Westermark B. Residual bodies and "aging" in cultured human glia cells. Effect of entrance into phase 3 and prolonged periods of confluence. Exp Eye Res. 1973 Apr;79(1):1–14. [PubMed] [Google Scholar]
- Buchheim W., Drenckhahn D., Lüllmann-Rauch R. Freeze-fracture studies of cytoplasmic inclusions occurring in experimental lipidosis as induced by amphiphilic cationic drugs. Biochim Biophys Acta. 1979 Oct 26;575(1):71–80. doi: 10.1016/0005-2760(79)90132-2. [DOI] [PubMed] [Google Scholar]
- Buckingham S., Heinemann H. O., Sommers S. C., McNary W. F. Phospholipid synthesis in the large pulmonary alveolar cell. Its relation to lung surfactants. Am J Pathol. 1966 Jun;48(6):1027–1041. [PMC free article] [PubMed] [Google Scholar]
- Buffaloe W. J., Johnson A. W., Sandifer M. G., Jr Total dosage of chlorpromazine and ocular opacities. Am J Psychiatry. 1967 Aug;124(2):250–251. doi: 10.1176/ajp.124.2.250. [DOI] [PubMed] [Google Scholar]
- Cain H., Kraus B. Lysosomale Pigmentablagerungen in der Niere alter Wistarratten. Virchows Arch B Cell Pathol. 1972;10(4):322–338. [PubMed] [Google Scholar]
- Cater B. R., Chapman D., Hawes S. M., Saville J. Lipid phase transitions and drug interactions. Biochim Biophys Acta. 1974 Aug 21;363(1):54–69. doi: 10.1016/0005-2736(74)90006-6. [DOI] [PubMed] [Google Scholar]
- Chatelain P., Berliner C., Ruysschaert J. M., Jaffé J. Effect of a diazafluoranthen derivative on phospholipases. A study at the air-water interface. Biochim Biophys Acta. 1976 Feb 6;419(3):540–546. doi: 10.1016/0005-2736(76)90264-9. [DOI] [PubMed] [Google Scholar]
- Chatelain P., May C. Abnormal microviscosity of lamellate cytosomes induced by a diazafluoranthen derivative. Biochem Pharmacol. 1979 Dec 1;28(23):3467–3470. doi: 10.1016/0006-2952(79)90088-1. [DOI] [PubMed] [Google Scholar]
- Chen I. L., Yates R. D. An ultrastructural study of opague cytoplasmic inclusions induced by triparanol treatment. Am J Anat. 1967 Nov;121(3):705–725. doi: 10.1002/aja.1001210314. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Cuault F., Vignais P. M. Characterization and subcellular localization of lipase activities in rat liver cell. Comparison with phospholipase A. Biochimie. 1974;56(2):275–288. doi: 10.1016/s0300-9084(74)80388-3. [DOI] [PubMed] [Google Scholar]
- Corrin B., King E. Experimental endogenous lipid pneumonia and silicosis. J Pathol. 1969 Feb;97(2):325–330. doi: 10.1002/path.1710970218. [DOI] [PubMed] [Google Scholar]
- Corrin B., Price A. B. Electron microscopic studies in desquamative interstitial pneumonia associated with asbestos. Thorax. 1972 May;27(3):324–331. doi: 10.1136/thx.27.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello J. F., Moriarty D. C., Branthwaite M. A., Turner-Warwick M., Corrin B. Diagnosis and management of alveolar proteinosis: the rôle of electron microscopy. Thorax. 1975 Apr;30(2):121–132. doi: 10.1136/thx.30.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowger M. L. Mechanism of bilirubin toxicity on tissue culture cells: factors that affect toxicity, reversibility by albumin, and comparison with other respiratory poisons and surfactants. Biochem Med. 1971 Feb;5(1):1–16. doi: 10.1016/0006-2944(71)90069-x. [DOI] [PubMed] [Google Scholar]
- D'Amico D. J., Kenyon K. R., Ruskin J. N. Amiodarone keratopathy: drug-induced lipid storage disease. Arch Ophthalmol. 1981 Feb;99(2):257–261. doi: 10.1001/archopht.1981.03930010259007. [DOI] [PubMed] [Google Scholar]
- Davidson J. M., Macleod W. M. Pulmonary alveolar proteinosis. Br J Dis Chest. 1969 Jan;63(1):13–28. doi: 10.1016/s0007-0971(69)80040-9. [DOI] [PubMed] [Google Scholar]
- De la Iglesia F. A., Feuer G., Takada A., Matsuda Y. Morphologic studies on secondary phospholipidosis in human liver. Lab Invest. 1974 Apr;30(4):539–549. [PubMed] [Google Scholar]
- Defrise-Quertain F., Chatelain P., Ruysschaert J. M. Phospholipase inactivation induced by an amino-piperazine derivative: a study at the lipid-water interface. J Pharm Pharmacol. 1978 Oct;30(10):608–612. doi: 10.1111/j.2042-7158.1978.tb13341.x. [DOI] [PubMed] [Google Scholar]
- Della Corte L., Gremigni D., Megazzini I., Mobilio R., Sgaragli G. P. Chlorimipramine-induced phospholipidosis: biochemical and pharmacokinetic observations [proceedings]. Br J Pharmacol. 1979 Nov;67(3):449P–450P. [PMC free article] [PubMed] [Google Scholar]
- Deodhar S. D., Bhagwat A. G. Desquamative interstitial pneumonia-like syndrome in rabbits. Produced experimentally by Freund's adjuvant. Arch Pathol. 1967 Jul;84(1):54–58. [PubMed] [Google Scholar]
- Desai R., Tetley T. D., Curtis C. G., Powell G. M., Richards R. J. Studies on the fate of pulmonary surfactant in the lung. Biochem J. 1978 Nov 15;176(2):455–462. doi: 10.1042/bj1760455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener R. M., Hsu B. Y. Effects of certain basic phenolic ethers on the rat fetus. Toxicol Appl Pharmacol. 1967 May;10(3):565–576. doi: 10.1016/0041-008x(67)90095-6. [DOI] [PubMed] [Google Scholar]
- Dietert S. E., Scallen T. J. An ultrastructural and biochemical study of the effects of three inhibitors of cholesterol biosynthesis upon murine adrenal gland and testis. Histochemical evidence for a lysosome response. J Cell Biol. 1969 Jan;40(1):44–60. doi: 10.1083/jcb.40.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D. Anterior polar cataract and lysosomal alterations in the lens of rats treated with the amphiphilic lipidosis-inducing drugs chloroquine and chlorphentermine. Virchows Arch B Cell Pathol. 1978 May 19;27(3):255–266. doi: 10.1007/BF02889000. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Jacobi B., Lüllmann-Rauch R. Corneal lipidosis in rats treated with amphiphilic cationic drugs. Arzneimittelforschung. 1983;33(6):827–831. [PubMed] [Google Scholar]
- Drenckhahn D., Kleine L., Lüllmann-Rauch R. Lysosomal alterations in cultured macrophages exposed to anorexigenic and psychotropic drugs. Lab Invest. 1976 Aug;35(2):116–123. [PubMed] [Google Scholar]
- Drenckhahn D., Lüllmann-Rauch R. Drug-induced experimental lipidosis in the nervous system. Neuroscience. 1979;4(6):697–612. doi: 10.1016/0306-4522(79)90001-0. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Lüllmann-Rauch R. Drug-induced retinal lipidosis: differential susceptibilities of pigment epithelium and neuroretina toward several amphiphilic cationic drugs. Exp Mol Pathol. 1978 Jun;28(3):360–371. doi: 10.1016/0014-4800(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Lüllmann-Rauch R. Experimental myopathy induced by amphiphilic cationic compounds including several psychotropic drugs. Neuroscience. 1979;4(4):549–562. doi: 10.1016/0306-4522(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Lüllmann-Rauch R. Lens opacities associated with lipidosis-like ultrastructural alterations in rats treated with chloroquine, chlorphentermine, or iprindole. Exp Eye Res. 1977 Jun;24(6):621–632. doi: 10.1016/0014-4835(77)90120-8. [DOI] [PubMed] [Google Scholar]
- Dubois-Dalq M., Buyse M., De Bruyne J., Van Tieghem N., Thiry L. Ultrastructural study of the action of aminopiperazines on transformed cell lines and on nervous system tissue culture. Exp Cell Res. 1973 Mar 15;77(1):303–311. doi: 10.1016/0014-4827(73)90581-8. [DOI] [PubMed] [Google Scholar]
- Dudognon P., Hauw J. J., de Baecque C., Escourolle R., Derrida J. P., Nick J. Neuropathie à l'amiodarone: lipidose médicamenteuse. Nouv Presse Med. 1979 May 12;8(21):1766–1767. [PubMed] [Google Scholar]
- Eichelbaum M., Hengstmann J. H., Dengler H. J. Das Verteilungsmuster des Chlorphentermins bei Ratte, Kaninchen und Schwein. Naunyn Schmiedebergs Arch Pharmakol. 1970;267(5):446–456. [PubMed] [Google Scholar]
- Eling T. E., Pickett R. D., Orton T. C., Anderson M. W. A study of the dynamics of imipramine accumulation in the isolated perfused rabbit lung. Drug Metab Dispos. 1975 Sep-Oct;3(5):389–399. [PubMed] [Google Scholar]
- Farr G. H., Harley R. A., Hennigar G. R. Desquamative interstitial pneumonia. An electron microscopic study. Am J Pathol. 1970 Sep;60(3):347–370. [PMC free article] [PubMed] [Google Scholar]
- Fedorko M. E. Effect of chloroquine on morphology of leukocytes and pancreatic exocrine cells from the rat. Lab Invest. 1968 Jan;18(1):27–37. [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G., Cohn Z. A. Autophagic vacuoles produced in vitro. I. Studies on cultured macrophages exposed to chloroquine. J Cell Biol. 1968 Aug;38(2):377–391. doi: 10.1083/jcb.38.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko M. Effect of chloroquine on morphology of cytoplasmic granules in maturing human leukocytes--an ultrastructural study. J Clin Invest. 1967 Dec;46(12):1932–1942. doi: 10.1172/JCI105683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S., Wang M. Y., Kaloyanides G. J. Aminoglycosides induce a phospholipidosis in the renal cortex of the rat: an early manifestation of nephrotoxicity. J Pharmacol Exp Ther. 1982 Mar;220(3):514–520. [PubMed] [Google Scholar]
- Ferin J. Alveolar macrophage mediated pulmonary clearance suppressed by drug-induced phospholipidosis. Exp Lung Res. 1982 Dec;4(1):1–10. doi: 10.3109/01902148209039245. [DOI] [PubMed] [Google Scholar]
- Flodh H., Magnusson G. Genesis of foam cells: study in rats after administration of Cloforex. Virchows Arch B Cell Pathol. 1973 Mar 30;12(4):360–366. doi: 10.1007/BF02894012. [DOI] [PubMed] [Google Scholar]
- Flodh H., Magnusson G., Magnusson O. Pulmonary foam cells in rats of different age. Z Versuchstierkd. 1974;16(4-5):299–312. [PubMed] [Google Scholar]
- Fowler B. A., Brooks R. E. Effects of the herbicide paraquat on the ultrastructure of mouse kidney. Am J Pathol. 1971 Jun;63(3):505–520. [PMC free article] [PubMed] [Google Scholar]
- France R., Gray M. E., Stone W. J., Swift L. L. Intracellular granules of the renal medulla in a case of potassium depletion due to renal potassium wasting. Electron microscopic comparison with renal medullary granules in the potassium-depleted rat. Am J Pathol. 1978 May;91(2):299–312. [PMC free article] [PubMed] [Google Scholar]
- Franken G., Lüllmann H., Siegfriedt A. The occurrence of huge cells in pulmonary alveoli of rats treated by an anorexic drug. Arzneimittelforschung. 1970 Mar;20(3):417–417. [PubMed] [Google Scholar]
- Fredman P., Klinghardt G. W., Nilsson O., Svennerholm L. Lipid accumulation in liver, spleen, lungs and kidneys of miniature-pigs after chloroquine treatment. Biochem J. 1982 Mar 1;201(3):581–588. doi: 10.1042/bj2010581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman P., Klinghardt G. W., Nilsson O., Svennerholm L. Lipid accumulation in liver, spleen, lungs and kidneys of miniature-pigs after chloroquine treatment. Biochem J. 1982 Mar 1;201(3):581–588. doi: 10.1042/bj2010581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freise J., Magerstedt P., Schmid K. Inhibition of phospholipase A2 by gabexate mesilate, camostate and aprotinine. Enzyme. 1983;30(3):209–212. doi: 10.1159/000469576. [DOI] [PubMed] [Google Scholar]
- Frentzen-Bertrams M., Debuch H. Production of bis(monoacylglycero)phosphate from phosphatidylglycerol in isolated liver lysosomes of chloroquine-pretreated rats. Hoppe Seylers Z Physiol Chem. 1981 Sep;362(9):1229–1236. doi: 10.1515/bchm2.1981.362.2.1229. [DOI] [PubMed] [Google Scholar]
- Frisch W., Lüllmann-Rauch R. Effects of several lipidosis-including drugs upon the area postrema and adjacent medullary nuclei of adult rats. I. Alterations is perikarya and dendrites. Acta Neuropathol. 1980;52(3):179–187. doi: 10.1007/BF00705806. [DOI] [PubMed] [Google Scholar]
- GOODWIN L. G., RICHARDS W. H., UDALL V. The toxicity of diaminodiphenoxyalkanes. Br J Pharmacol Chemother. 1957 Dec;12(4):468–474. doi: 10.1111/j.1476-5381.1957.tb00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gairola C., Matulionis D. H., Reasor M. J. Chlorphentermine-induced alterations in the lungs of vitamin E-deficient and supplemented rats: 1. Biochemical and morphometric analysis of the pulmonary response. Exp Mol Pathol. 1983 Jun;38(3):368–379. doi: 10.1016/0014-4800(83)90076-x. [DOI] [PubMed] [Google Scholar]
- Gaton E., Wolman M. Histochemical study on the pathogenesis of chlorocyclizine-induced pulmonary lipidosis. Histochemistry. 1979 Sep;63(2):203–207. doi: 10.1007/BF00644542. [DOI] [PubMed] [Google Scholar]
- Gautier M., Raulin J., Lapous D., Loriette C., Carreau J. P., Scotto J. Children sea-blue histiocytosis (2 cases) compared with phospholipidosis induced by 4-4' DET (4-4' p (diethylamino-2-ethoxy phenyl) 3-4 hexane) in rat. Biomedicine. 1977 Feb;26(1):52–60. [PubMed] [Google Scholar]
- Gefter W. B., Epstein D. M., Pietra G. G., Miller W. T. Lung disease caused by amiodarone, a new antiarrythmic agent. Radiology. 1983 May;147(2):339–344. doi: 10.1148/radiology.147.2.6836114. [DOI] [PubMed] [Google Scholar]
- Gerber N., Arnold K. The effect of diphenyl-piperazine compounds and other agents on diphenylhydantoin, zoxazolamine and hexobarbital metabolism. J Pharmacol Exp Ther. 1968 Nov;164(1):232–238. [PubMed] [Google Scholar]
- Glassy M. C., Ferrone S. Ultrastructural alterations in human lymphoblastoid B cell lines treated with tunicamycin. Am J Pathol. 1981 Apr;103(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Gleiser C. A., Bay W. W., Dukes T. W., Brown R. S., Read W. K., Pierce K. R. Study of chloroquine toxicity and a drug-induced cerebrospinal lipodystrophy in swine. Am J Pathol. 1968 Jul;53(1):27–45. [PMC free article] [PubMed] [Google Scholar]
- Gloster J., Heath D., Hasleton P., Harris P. Effect of chlorphentermine on the lipids of rat lungs. Thorax. 1976 Oct;31(5):558–564. doi: 10.1136/thx.31.5.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goormaghtigh E., Chatelain P., Caspers J., Ruysschaert J. M. Evidence of a specific complex between adriamycin and negatively-charged phospholipids. Biochim Biophys Acta. 1980 Mar 27;597(1):1–14. doi: 10.1016/0005-2736(80)90145-5. [DOI] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. E., Purmalis A., Purmalis B., Mathews J. Ultrastructural studies of the hepatic changes brought about by Clindamycin and Erythromycin in animals. Toxicol Appl Pharmacol. 1971 Jun;19(2):217–233. doi: 10.1016/0041-008x(71)90108-6. [DOI] [PubMed] [Google Scholar]
- Gray J. E., Weaver R. N., Stern K. F., Phillips W. A. Foam cell response in the lung and lymphatic tissues during long-term high-level treatment with erythromycin. Toxicol Appl Pharmacol. 1978 Sep;45(3):701–711. doi: 10.1016/0041-008x(78)90163-1. [DOI] [PubMed] [Google Scholar]
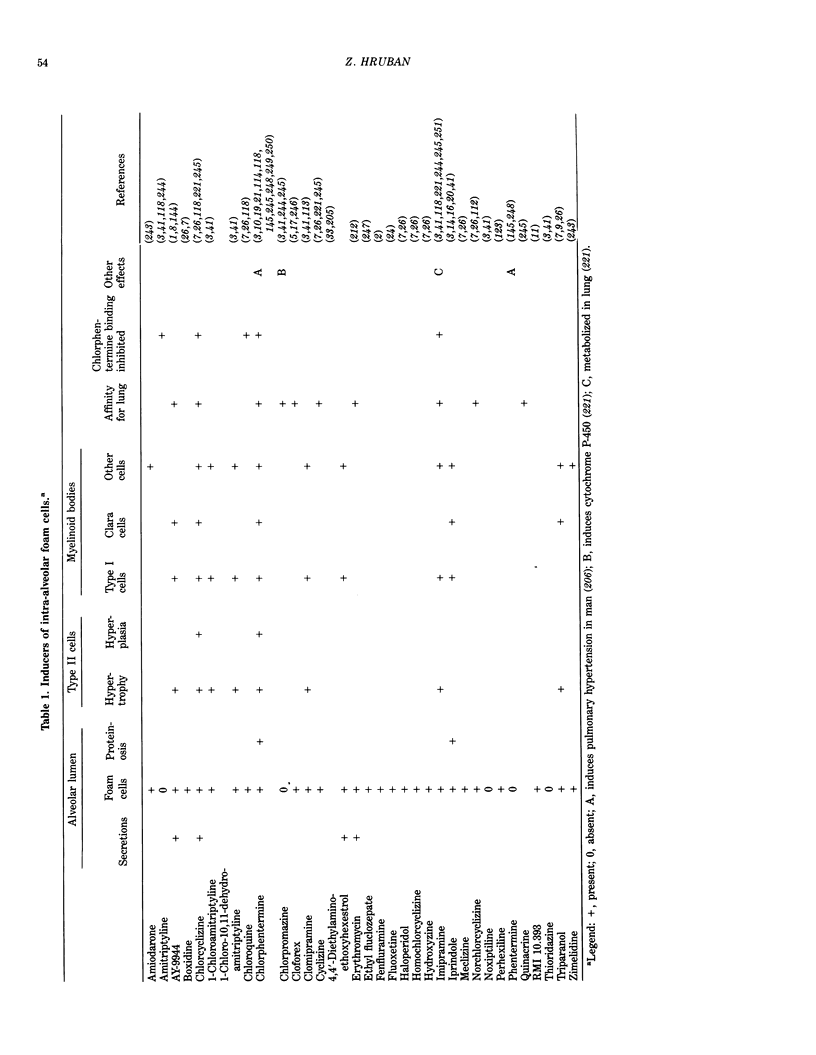
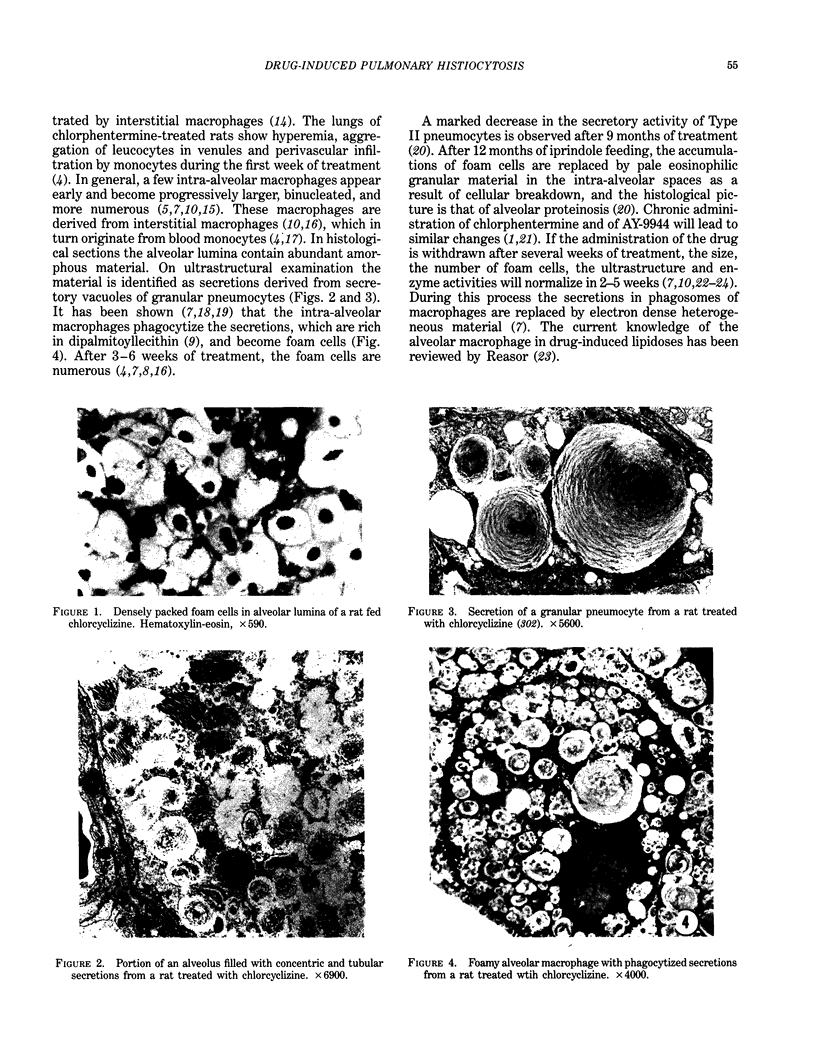
- Greselin E. An inhibitor of cholesterol biosynthesis and the alveolar macrophages. Can J Comp Med Vet Sci. 1966 May;30(5):121–126. [PMC free article] [PubMed] [Google Scholar]
- Gräbner R., Meerbach W. Morphological and biochemical alterations of the lung after application of chlorphentermine. Exp Pathol (Jena) 1979;17(6):303–311. doi: 10.1016/s0014-4908(79)80045-9. [DOI] [PubMed] [Google Scholar]
- HRUBAN Z., SPARGO B., SWIFT H., WISSLER R. W., KLEINFELD R. G. Focal cytoplasmic degradation. Am J Pathol. 1963 Jun;42:657–683. [PMC free article] [PubMed] [Google Scholar]
- Hallman M., Gluck L. Phosphatidylglycerol in lung surfactant. II. Subcellular distribution and mechanism of biosynthesis in vitro. Biochim Biophys Acta. 1975 Nov 21;409(2):172–191. doi: 10.1016/0005-2760(75)90152-6. [DOI] [PubMed] [Google Scholar]
- Hauw J. J., Boutry J. M., Albouz S., Harpin M. L., Baudrimont M., Escourolle R., Baumann N. Perhexiline maleate-induced lipidosis in cultured human fibroblasts: cell kinetics, ultrastructural and biochemical studies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;34(3):239–249. doi: 10.1007/BF02892421. [DOI] [PubMed] [Google Scholar]
- Hauw J. J., Mussini J. M., Boutry J. M., Escourolle R., Pollet S., Albouz S., Harpin M. L., Baumann N. Perhexiline maleate induced lipidosis in human peripheral nerve and tissue culture: ultrastructural and biochemical changes. Clin Toxicol. 1981 Dec;18(12):1405–1409. doi: 10.3109/15563658108990349. [DOI] [PubMed] [Google Scholar]
- Heath D., Smith P., Hasleton P. S. Effects of chlorphentermine on the rat lung. Thorax. 1973 Sep;28(5):551–558. doi: 10.1136/thx.28.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendelman W. J. A morphologic study of the effects of LSD on neurons in cultures of cerebelum. J Neuropathol Exp Neurol. 1972 Jul;31(3):411–432. doi: 10.1097/00005072-197207000-00002. [DOI] [PubMed] [Google Scholar]
- Henkart M. Light-induced changes in the structure of pigmented granules in aplysia neurons. Science. 1975 Apr 11;188(4184):155–157. doi: 10.1126/science.1114345. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Wright N. A., Stewart J. A. Experimental alveolar lipo-proteinosis following the inhalation of silica. J Pathol. 1970 Aug;101(4):293–307. doi: 10.1002/path.1711010402. [DOI] [PubMed] [Google Scholar]
- Hildebrand J., Thys O., Gérin Y. Alterations of rat liver lysosomes and smooth endoplasmic reticulum induced by the diazafluoranthen derivative AC-3579. II. Effects of the drug on phospholipid metabolism. Lab Invest. 1973 Jan;28(1):83–86. [PubMed] [Google Scholar]
- Hirst L. W., Sanborn G., Green W. R., Miller N. R., Heath W. D. Amodiaquine ocular changes. Arch Ophthalmol. 1982 Aug;100(8):1300–1304. doi: 10.1001/archopht.1982.01030040278015. [DOI] [PubMed] [Google Scholar]
- Hoenig V., Werner F. Effect of perhexiline maleate on lipid metabolism in the rat. Arzneimittelforschung. 1979;29(9):1395–1398. [PubMed] [Google Scholar]
- Hoffmann E. O., Lamberty J., Pizzolato P., Coover J. The ultrastructure of acute silicosis. Arch Pathol. 1973 Aug;96(2):104–107. [PubMed] [Google Scholar]
- Hook G. E., Bend J. R. Pulmonary metabolism of xenobiotics. Life Sci. 1976 Feb 1;18(3):279–290. doi: 10.1016/0024-3205(76)90056-4. [DOI] [PubMed] [Google Scholar]
- Hook G. E., DiAugustine R. P. Secretory cells of the peripheral pulmonary epithelium as targets for toxic agents. Environ Health Perspect. 1976 Aug;16:147–156. doi: 10.1289/ehp.7616147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., Hall L. B. Inhibition of kidney lysosomal phospholipases A and C by aminoglycoside antibiotics: possible mechanism of aminoglycoside toxicity. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1663–1667. doi: 10.1073/pnas.79.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler K. Y., Matsuzawa Y. Studies on the mechanism of drug-induced lipidosis. Cationic amphiphilic drug inhibition of lysosomal phospholipases A and C. Biochem Pharmacol. 1981 May 15;30(10):1121–1126. doi: 10.1016/0006-2952(81)90451-2. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Richman D. D. Studies on the mechanism of phospholipid storage induced by amantadine and chloroquine in Madin Darby canine kidney cells. Biochem Pharmacol. 1982 Dec 1;31(23):3795–3799. doi: 10.1016/0006-2952(82)90295-7. [DOI] [PubMed] [Google Scholar]
- Houghton D. C., Hartnett M., Campbell-Boswell M., Porter G., Bennett W. A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am J Pathol. 1976 Mar;82(3):589–612. [PMC free article] [PubMed] [Google Scholar]
- Houghton D. C., Plamp C. E., 3rd, DeFehr J. M., Bennett W. M., Porter G., Gilbert D. Gentamicin and tobramycin nephrotoxicity. A morphologic and functional comparison in the rat. Am J Pathol. 1978 Oct;93(1):137–152. [PMC free article] [PubMed] [Google Scholar]
- Hruban Z. Pulmonary changes induced by amphophilic drugs. Environ Health Perspect. 1976 Aug;16:111–118. doi: 10.1289/ehp.7616111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruban Z., Rubenstein A. H., Slesers A. Alterations in pancreatic beta cells induced by cyclizine. Lab Invest. 1972 Mar;26(3):270–277. [PubMed] [Google Scholar]
- Hruban Z., Slesers A., Aschenbrenner I. Pulmonary intra-alveolar histiocytosis induced by drugs. Toxicol Appl Pharmacol. 1973 Sep;26(1):72–85. doi: 10.1016/0041-008x(73)90087-2. [DOI] [PubMed] [Google Scholar]
- Hruban Z., Slesers A., Hopkins E. Drug-induced and naturally occurring myeloid bodies. Lab Invest. 1972 Jul;27(1):62–70. [PubMed] [Google Scholar]
- Hruban Z., Swift H., Slesers A. Effect of triparanol and diethanolamine on the fine structure of hepatocytes and pancreatic acinar cells. Lab Invest. 1965 Sep;14(9):1652–1672. [PubMed] [Google Scholar]
- Hruban Z., Tavoloni N., Reed J. S., Boyer J. L. Ultrastructural changes during cholestasis induced by chlorpromazine in the isolated perfused rat liver. Virchows Arch B Cell Pathol. 1978 Feb 14;26(4):289–305. doi: 10.1007/BF02889557. [DOI] [PubMed] [Google Scholar]
- Ito S., Tsukada Y. Clinico-pathological and electron microscopical studies on a coronary dilating agent: 4-4'-diethylaminoethoxyhexestrol-induced liver injuries. Acta Hepatogastroenterol (Stuttg) 1973 May-Jun;20(3):204–215. [PubMed] [Google Scholar]
- Jain M. K., Wu N. Y., Wray L. V. Drug-induced phase change in bilayer as possible mode of action of membrane expanding drugs. Nature. 1975 Jun 5;255(5508):494–496. doi: 10.1038/255494a0. [DOI] [PubMed] [Google Scholar]
- James-Kracke M. R., Sloane B. F., Shuman H., Somlyo A. P. Lysosomal composition in cultured vascular smooth muscle cells: electron probe analysis. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6461–6465. doi: 10.1073/pnas.76.12.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. J., Suzuki K. Morphological changes in CNS of rats treated with perhexiline maleate (pexid). Acta Neuropathol. 1978 Jun 30;42(3):159–164. doi: 10.1007/BF00690352. [DOI] [PubMed] [Google Scholar]
- Junger E., Reinauer H. Liquid crystalline phases of hydrated phosphatidylethanolamine. Biochim Biophys Acta. 1969 Jul 15;183(2):304–308. doi: 10.1016/0005-2736(69)90086-8. [DOI] [PubMed] [Google Scholar]
- KUNTZMAN R., KLUTCH A., TSAI I., BURNS J. J. PHYSIOLOGICAL DISTRIBUTION AND METABOLIC INACTIVATION OF CHLORCYCLIZINE AND CYCLIZINE. J Pharmacol Exp Ther. 1965 Jul;149:29–35. [PubMed] [Google Scholar]
- Kacew S. Alterations in newborn and adult rat lung morphology and phospholipid levels after chlorcyclizine or chlorphentermine treatment. Toxicol Appl Pharmacol. 1982 Aug;65(1):100–108. doi: 10.1016/0041-008x(82)90367-2. [DOI] [PubMed] [Google Scholar]
- Kacew S., Narbaitz R. A comparative ultrastructural and biochemical study between the effects of chlorphentermine and phentermine on rat lung. Exp Mol Pathol. 1977 Aug;27(1):106–120. doi: 10.1016/0014-4800(77)90023-5. [DOI] [PubMed] [Google Scholar]
- Kacew S., Narbaitz R., Dubas T. C. Biochemical and morphologic investigation of the influence of chlorphentermine and subsequent withdrawal on newborn rat lung. Toxicol Appl Pharmacol. 1979 Feb;47(2):185–191. doi: 10.1016/0041-008x(79)90311-9. [DOI] [PubMed] [Google Scholar]
- Kacew S., Narbaitz R. Early metabolic alterations in pulmonary tissue following administration of toxic agents. Fed Proc. 1978 Sep;37(11):2489–2495. [PubMed] [Google Scholar]
- Kacew S., Narbaitz R., Ruddick J. A., Villeneuve D. C. Role of drug metabolism in protection against chlorphentermine-induced pulmonary phospholipidosis in adult rat. Exp Mol Pathol. 1981 Aug;35(1):98–107. doi: 10.1016/0014-4800(81)90010-1. [DOI] [PubMed] [Google Scholar]
- Kacew S., Narbaitz R. The effect of phenobarbital on chlorphentermine-induced lipidosis-like alterations in renal tissue of adult and newborn rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;36(1):59–63. doi: 10.1007/BF02912055. [DOI] [PubMed] [Google Scholar]
- Kalina M., Bubis J. J. Myeloid bodies formation in triparanol treated cultured cells. Virchows Arch B Cell Pathol. 1975 Dec 19;19(4):349–357. doi: 10.1007/BF02889378. [DOI] [PubMed] [Google Scholar]
- Karabelnik D., Zbinden G., Baumgartner E. Drug-induced foam cell reactions in rats. I. Histopathologic and cytochemical observations after treatment with chlorphentermine, RMI 10.393 and Ro 4-4318. Toxicol Appl Pharmacol. 1974 Feb;27(2):395–407. doi: 10.1016/0041-008x(74)90210-5. [DOI] [PubMed] [Google Scholar]
- Karabelnik D., Zbinden G. Drug-induced foam cell reactions in rats, II. Chemical analysis of lipids stored in lungs and foam cells after treatment with chlorphentermine, 5-[p-(fluoren-9-ylidenemethyl)phenyl]-2-piperidineethanol (RMI 10.393) and 1-chloramitriptyline. Hoppe Seylers Z Physiol Chem. 1975 Jul;356(7):1151–1160. doi: 10.1515/bchm2.1975.356.2.1151. [DOI] [PubMed] [Google Scholar]
- Karabelnik D., Zbinden G. Palmitic acid-1-14C incorporation and turnover in lung phospholipids of rats treated with chlorphentermine, RMI 10.393 and Ro 4-4318. Arch Toxicol. 1976 Jun 8;35(3):163–174. doi: 10.1007/BF00293563. [DOI] [PubMed] [Google Scholar]
- Kasama K., Yoshida K., Takeda S., Akeda S., Kawai K. Bis-(monoacylglyceryl)phosphate and acyl phosphatidylglycerol isolated from human livers of lipidosis induced by 4,4'-diethylaminoethoxyhexesterol. Lipids. 1974 Apr;9(4):235–243. doi: 10.1007/BF02532199. [DOI] [PubMed] [Google Scholar]
- Kasama K., Yoshida K., Takeda S., Tsujimura R., Hasegawa S. Inhibition of acid esterase in rat liver by 4,4'-diethylamino-ethoxyhexestrol. Lipids. 1976 Oct;11(10):718–721. doi: 10.1007/BF02533044. [DOI] [PubMed] [Google Scholar]
- Keeffe E. B., Blankenship N. M., Scharschmidt B. F. Alteration of rat liver plasma membrane fluidity and ATPase activity by chlorpromazine hydrochloride and its metabolites. Gastroenterology. 1980 Aug;79(2):222–231. [PubMed] [Google Scholar]
- Kikkawa Y., Motoyama E. K. Effect of AY-9944, a cholesterol biosynthesis inhibitor, on fetal lung development and on the development of type II alveolar epithelial cells. Lab Invest. 1973 Jan;28(1):48–54. [PubMed] [Google Scholar]
- Kikkawa Y., Suzuki K. Alteration of cellular and acellular alveolar and bronchiolar walls produced by hypocholesteremic drug AY9944. Lab Invest. 1972 Apr;26(4):441–447. [PubMed] [Google Scholar]
- Kim S. U. Effects of the cholesterol biosynthesis inhibitor ay9944 on organotypic cultures ofmouse spinal cord. Retarded myelinogenesis and induction of cytoplasmic inclusions. Lab Invest. 1975 Jun;32(6):720–728. [PubMed] [Google Scholar]
- Kirby T. J. Cataracts produced by triparanol. (MER-29). Trans Am Ophthalmol Soc. 1967;65:494–543. [PMC free article] [PubMed] [Google Scholar]
- Kitani T., Yamamoto A., Adachi S., Tako H., Imanaka T. [Drug-induced lipidosis. IV. Morphological aspect of leukocytes and bone marrow cells in human cases and animal experiments induced by 4,4'-diethylaminoethoxy-hexestrol]. Nihon Ketsueki Gakkai Zasshi. 1972 Apr;35(2):131–145. [PubMed] [Google Scholar]
- Klinghardt G. W., Fredman P., Svennerholm L. Chloroquine intoxication induces ganglioside storage in nervous tissue: a chemical and histopathological study of brain, spinal cord, dorsal root ganglia, and retinal in the miniature pig. J Neurochem. 1981 Oct;37(4):897–908. doi: 10.1111/j.1471-4159.1981.tb04477.x. [DOI] [PubMed] [Google Scholar]
- Kosek J. C., Mazze R. I., Cousins M. J. Nephrotoxicity of gentamicin. Lab Invest. 1974 Jan;30(1):48–57. [PubMed] [Google Scholar]
- Kuhn C., Györkey F., Levine B. E., Ramirez-Rivera J. Pulmonary alveolar proteinosis. A study using enzyme histochemistry, electron microscopy, and surface tension measurement. Lab Invest. 1966 Feb;15(2):492–509. [PubMed] [Google Scholar]
- Kunze H., Hesse B., Bohn E. Effects of antimalarial drugs on several rat-liver lysosomal enzymes involved in phosphatidylethanolamine catabolism. Biochim Biophys Acta. 1982 Oct 14;713(1):112–117. doi: 10.1016/0005-2760(82)90173-4. [DOI] [PubMed] [Google Scholar]
- Kunze H., Nahas N., Traynor J. R., Wurl M. Effects of local anaesthetics on phospholipases. Biochim Biophys Acta. 1976 Jul 20;441(1):93–102. doi: 10.1016/0005-2760(76)90284-8. [DOI] [PubMed] [Google Scholar]
- Kvarstein B., Stormorken H. Influence of acetylsalicylic acid, butazolidine, colchicine, hydrocortisone, chlorpromazine and imipramine on the phagocytosis of polystyrene latex particles by human leucocytes. Biochem Pharmacol. 1971 Jan;20(1):119–124. doi: 10.1016/0006-2952(71)90477-1. [DOI] [PubMed] [Google Scholar]
- Kühnel W. Die Glandulae rectales (Proctodaealdrüsen) des Kaninchens. Elektronenmikdroskopische Untersuchungen. Z Zellforsch Mikrosk Anat. 1971;122(4):574–583. [PubMed] [Google Scholar]
- Lageron A., Saffroy M. Modifications morphologiques histochimiques biochimiques induites par l'homochlorcyclizine chez le rat. Acta Histochem. 1982;71(1):103–110. [PubMed] [Google Scholar]
- Lageron A., Scotto J., Gautier M. Effects of Pexid on liver cell cultures. Ultrastructural and histoenzymological studies. Eur J Clin Pharmacol. 1981;19(6):417–421. doi: 10.1007/BF00548585. [DOI] [PubMed] [Google Scholar]
- Laurent G., Hildebrand J., Thys O. Alterations of rat liver lysosomes and somooth endoplasmic reticulum induced by the diazafluoranthen derivative AC-3579. III. Mechanism and site of action. Lab Invest. 1975 May;32(5):580–584. [PubMed] [Google Scholar]
- Le Beux Y., Hetenyi G., Jr, Phillips M. J. Mitochondrial myelin-like figures: a non-specific reactive process of mitochondrial phospholipid membranes to several stimuli. Z Zellforsch Mikrosk Anat. 1969;99(4):491–506. doi: 10.1007/BF00340941. [DOI] [PubMed] [Google Scholar]
- Le Gall J. Y., Guillouzo A., Glaise D., Deugnier Y., Messner M., Bourel M. Perhexiline maleate toxicity on human liver cell lines. Gut. 1980 Nov;21(11):977–984. doi: 10.1136/gut.21.11.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson G. A., Biedenbach S. A., Chan K. Y., Gibson J. P., Wright G. J. Decrease in the activity of the drug-metabolizing enzymes of rat liver following the administration of tilorone hydrochloride. Drug Metab Dispos. 1976 May-Jun;4(3):232–238. [PubMed] [Google Scholar]
- Lehnert B. E., Ferin J. Particle binding, phagocytosis, and plastic substrate adherence characteristics of alveolar macrophages from rats acutely treated with chlorphentermine. J Reticuloendothel Soc. 1983 Apr;33(4):293–303. [PubMed] [Google Scholar]
- Lhermitte F., Fardeau M., Chedru F., Mallecourt J. Polyneuropathy after perhexiline maleate therapy. Br Med J. 1976 May 22;1(6020):1256–1256. doi: 10.1136/bmj.1.6020.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liveson J. A., Gardner J., Bornstein M. B. Tissue culture studies of possible uremic neurotoxins: myoinositol. Kidney Int. 1977 Aug;12(2):131–136. doi: 10.1038/ki.1977.90. [DOI] [PubMed] [Google Scholar]
- Lockard V. G., Watson S. A., Siraj M. Y., Hayes A. W., O'Neal R. M. Rubratoxin B hepatotoxicity: an electron microscopic study. Exp Mol Pathol. 1981 Feb;34(1):94–109. doi: 10.1016/0014-4800(81)90039-3. [DOI] [PubMed] [Google Scholar]
- Loker E., Scallen T. J., Dietert S. E. Phenobarbital-induced proliferation of smooth endoplasmic reticulum after administration of triparanol. Anat Rec. 1970 Oct;168(2):221–232. doi: 10.1002/ar.1091680208. [DOI] [PubMed] [Google Scholar]
- Luft F. C., Yum M. N., Walker P. D., Kleit S. A. Gentamicin gradient patterns and morphological changes in human kidneys. Nephron. 1977;18(3):167–174. doi: 10.1159/000180811. [DOI] [PubMed] [Google Scholar]
- Lúllmann-Rauch R., Reil G. H. Fenfluramine-induced ultrastructural alterations in tissues of rats and guinea pigs. Naunyn Schmiedebergs Arch Pharmacol. 1974;285(2):175–184. doi: 10.1007/BF00501152. [DOI] [PubMed] [Google Scholar]
- Lüllmann Rauch R., Reil G. H. Chlorphentermine-induced lipidosislike ultrastructural alterations in lungs and adrenal glands of several species. Toxicol Appl Pharmacol. 1974 Dec;30(3):408–421. doi: 10.1016/0041-008x(74)90263-4. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R. Chlorphentermine-induced ultrastructural alterations in foetal tissues. Virchows Arch B Cell Pathol. 1973 Mar 30;12(4):295–302. doi: 10.1007/BF02894007. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R. Experimentally induced lipidosis in rat retinal pigment epithelium. A brief review. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981;215(4):297–303. doi: 10.1007/BF00407668. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R. Histochemical evidence for lysosomal storage of acid glycosaminoglycans in splenic cells of rats treated with tilorone. Histochemistry. 1982;76(1):71–87. doi: 10.1007/BF00493287. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R., Nässberger L. Citalopram-induced generalized lipidosis in rats. Acta Pharmacol Toxicol (Copenh) 1983 Mar;52(3):161–167. doi: 10.1111/j.1600-0773.1983.tb01080.x. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R., Pietschmann N. Lipidosis-like cellular alterations in lymphatic tissues of chlorphentermine-treated animals. Virchows Arch B Cell Pathol. 1974;15(4):295–308. doi: 10.1007/BF02889345. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R., Reil G. H., Rossen E., Seiler K. U. The ultrastructure of rat lung changes induced by an anorectic drug (chlorphentermine). Virchows Arch B Cell Pathol. 1972;11(2):167–181. doi: 10.1007/BF02889396. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R. Retinal lipidosis in albino rats treated with chlorphentermine and with tricyclic antidepressants. Acta Neuropathol. 1976 May 18;35(1):55–67. doi: 10.1007/BF00688943. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R., Scheid D. Intraalveolar foam cells associated with lipidosis-like alterations in lung and liver of rats treated with tricyclic psychotropic drugs. Virchows Arch B Cell Pathol. 1975 Nov 21;19(3):255–268. doi: 10.1007/BF02889372. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R., Stoermer B. Generalized lipidosis in newborn rats and Guinea pigs induced during prenatal development by administration of amphiphilic drugs to pregnant animals. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39(1):59–73. doi: 10.1007/BF02892837. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Lüllmann-Rauch R. Perhexiline induces generalized lipidosis in rats. Klin Wochenschr. 1978 Mar 15;56(6):309–310. doi: 10.1007/BF01489178. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Lüllmann-Rauch R., Reil G. H. A comparative ultrastructural study of the effects of chlorphentermine and triparanol in rat lung and adrenal gland. Virchows Arch B Cell Pathol. 1973 Jan 31;12(2):91–103. doi: 10.1007/BF02893989. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Lüllmann-Rauch R. Tamoxifen-induced generalized lipidosis in rats subchronically treated with high doses. Toxicol Appl Pharmacol. 1981 Oct;61(1):138–146. doi: 10.1016/0041-008x(81)90014-4. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Lüllmann-Rauch R., Wassermann O. Drug-induced phospholipidoses. II. Tissue distribution of the amphiphilic drug chlorphentermine. CRC Crit Rev Toxicol. 1975 Nov;4(2):185–218. doi: 10.1080/10408447509164014. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Mösinger E. U. Increased lysosomal enzyme activities in tissues of rats suffering from chlorphentermine induced lipidosis. Biochem Pharmacol. 1979 Apr 1;28(7):1015–1016. doi: 10.1016/0006-2952(79)90296-x. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Plösch H., Ziegler A. Ca replacement by cationic amphiphilic drugs from lipid monolayers. Biochem Pharmacol. 1980 Nov 1;29(21):2969–2974. doi: 10.1016/0006-2952(80)90046-5. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Wehling M. The binding of drugs to different polar lipids in vitro. Biochem Pharmacol. 1979 Dec 1;28(23):3409–3415. doi: 10.1016/0006-2952(79)90080-7. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Magnusson O. Cloforex-induced pulmonary changes in rats. Beitr Pathol. 1972 Apr;146(1):79–88. [PubMed] [Google Scholar]
- Marchlinski F. E., Gansler T. S., Waxman H. L., Josephson M. E. Amiodarone pulmonary toxicity. Ann Intern Med. 1982 Dec;97(6):839–845. doi: 10.7326/0003-4819-97-6-839. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epithelial cells of presumed type II cell origin. Biochim Biophys Acta. 1980 Jan 18;617(1):36–50. doi: 10.1016/0005-2760(80)90222-2. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Effects of chloroquine and 4,4'-bis(diethylaminoethoxy)alpha, beta-diethyldiphenylethane on the incorporation of [3H]glycerol into the phospholipids of rat liver lysosomes and other subcellular fractions, in vivo. Biochim Biophys Acta. 1980 Dec 5;620(3):592–602. doi: 10.1016/0005-2760(80)90151-4. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4'-bis(diethylaminoethoxy) alpha, beta-diethyldiphenylethane. J Biol Chem. 1980 Jun 10;255(11):5190–5194. [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Studies on drug-induced lipidosis: subcellular localization of phospholipid and cholesterol in the liver of rats treated with chloroquine or 4,4'-bis (diethylaminoethoxy)alpha, beta-diethyldiphenylethane. J Lipid Res. 1980 Feb;21(2):202–214. [PubMed] [Google Scholar]
- Matsuzawa Y., Yamamoto A., Adachi S., Nishikawa M. Studies on drug-induced lipidosis. VIII. Correlation between drug accumulation and acidic phospholipids. J Biochem. 1977 Nov;82(5):1369–1377. doi: 10.1093/oxfordjournals.jbchem.a131824. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Yokomura T., Ishikawa K., Adachi S., Yamamoto A. Studies on drug-induced lipidosis. VI. Identification and determination of the drug and its metabolite in lipidosis induced by 4,4'-diethylaminoethoxyhexestrol. J Biochem. 1972 Sep;72(3):615–621. doi: 10.1093/oxfordjournals.jbchem.a129939. [DOI] [PubMed] [Google Scholar]
- Matulionis D. H., Gairola C., Reasor M. J. Chlorphentermine-induced alterations in the lungs of vitamin E-deficient and -supplemented rats: quantitative ultrastructural analysis of Type II Cell response. Exp Mol Pathol. 1983 Aug;39(1):80–88. doi: 10.1016/0014-4800(83)90042-4. [DOI] [PubMed] [Google Scholar]
- Mazue G., Berthe J., Newmann A. J., Brunaud M. A toxicologic evaluation of ethyl fluclozepate (CM 6912). Int J Clin Pharmacol Ther Toxicol. 1981 Oct;19(10):453–472. [PubMed] [Google Scholar]
- McKeown C. A., Swartz M., Blom J., Maggiano J. M. Tamoxifen retinopathy. Br J Ophthalmol. 1981 Mar;65(3):177–179. doi: 10.1136/bjo.65.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara T. E., Goodloe S., Butkus D. E. Myeloid bodies in patients without clinical Fabry's disease. Arch Pathol Lab Med. 1980 Jan;104(1):14–16. [PubMed] [Google Scholar]
- McPherson J. R., Shorter R. G. Intestinal lesions associated with triparanol. A clinical and experimental study. Am J Dig Dis. 1965 Dec;10(12):1024–1033. doi: 10.1007/BF02233376. [DOI] [PubMed] [Google Scholar]
- Mellors A., Tappel A. L. Hydrolysis of phospholipids by a lysosomal enzyme. J Lipid Res. 1967 Sep;8(5):479–485. [PubMed] [Google Scholar]
- Michell R. H., Allan D., Bowley M., Brindley D. N. A possible metabolic explanation for drug-induced phospholipidosis. J Pharm Pharmacol. 1976 Apr;28(4):331–332. doi: 10.1111/j.2042-7158.1976.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Mielke H., Seiler K. U., Stumpf U., Wassermann O. Uber eine Beziehung zwischen dem Serotoninstoffwechsel und der pulmonalen Hypertonie bei Ratten nach Gabe verschiedener Anorektika. Z Kardiol. 1973 Dec;62(12):1090–1098. [PubMed] [Google Scholar]
- Miller D. K., Lenard J. Antihistaminics, local anesthetics, and other amines as antiviral agents. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3605–3609. doi: 10.1073/pnas.78.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin R. F., Ilett K. F., Madsen B. W. Chlorphentermine binding in rat lung subcellular fractions and its displacement by desmethylimipramine. Biochem Pharmacol. 1979 Aug 1;28(15):2273–2278. doi: 10.1016/0006-2952(79)90689-0. [DOI] [PubMed] [Google Scholar]
- Minchin R. F., Madsen B. W., Ilett K. F. Effect of desmethylimipramine on the kinetics of chlorphentermine accumulation in isolated perfused rat lung. J Pharmacol Exp Ther. 1979 Dec;211(3):514–518. [PubMed] [Google Scholar]
- Mizuno G. R., Chapman C. J., Chipault J. R., Pfeiffer D. R. Lipid composition and (Na+ + K+)-ATPase activity in rat lens during triparanol-induced cataract formation. Biochim Biophys Acta. 1981 Jun 9;644(1):1–12. doi: 10.1016/0005-2736(81)90052-3. [DOI] [PubMed] [Google Scholar]
- Montgomery M. R., Furry J. M., Reasor M. J. Chlorphentermine inhibits oxidative energy metabolism in rat lung slices. Toxicol Appl Pharmacol. 1982 Aug;65(1):63–68. doi: 10.1016/0041-008x(82)90362-3. [DOI] [PubMed] [Google Scholar]
- Mussini J. M., Hauw J. J., Escourolle R. Etude en microscopie électronique des lésions nerveuses, musculaires et cutanées déterminées par le maléate de perhexiline. Acta Neuropathol. 1977 Apr 29;38(1):53–59. doi: 10.1007/BF00691277. [DOI] [PubMed] [Google Scholar]
- NARROD S. A., WILK A. L., KING C. T. METABOLISM OF MECLIZINE IN THE RAT. J Pharmacol Exp Ther. 1965 Mar;147:380–384. [PubMed] [Google Scholar]
- Naimark A. Cellular dynamics and lipid metabolism in the lung. Fed Proc. 1973 Sep;32(9):1967–1971. [PubMed] [Google Scholar]
- Nick J., Dudognon P., Escourolle R., Bakouche P., Nicolle M. H., Reignier A., Hauw J. J., Ermidou S., Pollet S., Baumann N. Manifestations neurologiques en rapport avec le traitement par le maléate de perhexiline. Etude clinique d'une série de 10 cas. Documents neuropathologiques, pharmacocinétiques et biochimiques. Rev Neurol (Paris) 1978 Feb;134(2):103–114. [PubMed] [Google Scholar]
- Nilsson O., Fredman P., Klinghardt G. W., Dreyfus H., Svennerholm L. Chloroquine-induced accumulation of gangliosides and phospholipids in skeletal muscles. Quantitative determination and characterization of stored lipids. Eur J Biochem. 1981 Jun 1;116(3):565–571. doi: 10.1111/j.1432-1033.1981.tb05373.x. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya Y., Angevine L. S., Mehendale H. M. Effect of drug-induced phospholipidosis on pulmonary disposition of pneumophilic drugs. Drug Metab Dispos. 1983 Jan-Feb;11(1):25–30. [PubMed] [Google Scholar]
- Pappu A. S., Hauser G. Alterations of phospholipid metabolism in rat cerebral cortex mince induced by cationic amphiphilic drugs. J Neurochem. 1981 Oct;37(4):1006–1014. doi: 10.1111/j.1471-4159.1981.tb04488.x. [DOI] [PubMed] [Google Scholar]
- Pappu A. S., Hauser G. Phospholipid metabolism changes in rat tissues in vitro after injections of propranolol. J Pharmacol Exp Ther. 1982 Jul;222(1):109–115. [PubMed] [Google Scholar]
- Parwaresch R., Reil G. H., Seiler K. U. Uber die Tier- und Organspezifität morphologischer Veränderungen nach chronischer Chlorphentermingabe. Res Exp Med (Berl) 1973 Nov 23;161(4):272–288. doi: 10.1007/BF01851451. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Manian A. A., Harcus J. L., Hall D., Hewlett E. L. Lethal effect of phenothiazine neuroleptics on the pathogenic protozoan Leishmania donovani. Science. 1982 Jul 23;217(4557):369–371. doi: 10.1126/science.6124040. [DOI] [PubMed] [Google Scholar]
- Perez del Cerro M., Snider R. S. Studies on Dilantin intoxication. I. Ultrastructural analogies with the lipoidoses. Neurology. 1967 May;17(5):452–466. doi: 10.1212/wnl.17.5.452. [DOI] [PubMed] [Google Scholar]
- Pessayre D., Bichara M., Degott C., Potet F., Benhamou J. P., Feldmann G. Perhexiline maleate-induced cirrhosis. Gastroenterology. 1979 Jan;76(1):170–177. [PubMed] [Google Scholar]
- Pieterse A. S., Rowland R., Dunn D. Perhexiline maleate induced cirrhosis. Pathology. 1983 Apr;15(2):201–203. doi: 10.3109/00313028309084714. [DOI] [PubMed] [Google Scholar]
- Plantavid M., Chap H., Lloveras J., Douste-Blazy L. Cationic amphiphilic drugs as a potential tool for modifying phospholipids of tumor cells. An in vitro study of chlorpromazine effects on Krebs II ascites cells. Biochem Pharmacol. 1981 Feb 15;30(4):293–297. doi: 10.1016/0006-2952(81)90057-5. [DOI] [PubMed] [Google Scholar]
- Quennedey A., Brossut R. Les glandes mandibulaires de Blaberus craniifer burm. (Dictyoptera, Blaberidae) developpement, structure et fonctionnement. Tissue Cell. 1975;7(3):503–517. doi: 10.1016/0040-8166(75)90022-1. [DOI] [PubMed] [Google Scholar]
- ROSEN S. H., CASTLEMAN B., LIEBOW A. A. Pulmonary alveolar proteinosis. N Engl J Med. 1958 Jun 5;258(23):1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- Ramsey R. B., Fischer V. W. The biochemical and morphological response of hydrolytic enzymes in the developing brain to hypocholesterolemic agents. Acta Neuropathol. 1980;49(2):89–94. doi: 10.1007/BF00690747. [DOI] [PubMed] [Google Scholar]
- Rawlins F. A., Uzman B. G. Effect of AY-9944, a cholesterol biosynthesis inhibitor, on peripheral nerve myelination. Lab Invest. 1970 Aug;23(2):184–189. [PubMed] [Google Scholar]
- Read W. K., Bay W. W. Basic cellular lesion in chloroquine toxicity. Lab Invest. 1971 Mar;24(3):246–259. [PubMed] [Google Scholar]
- Reasor M. J. Cumene hydroperoxide-mediated lipid peroxidation in rat alveolar macrophages following induction of phospholipidosis with chlorphentermine. Toxicology. 1980;18(2):159–168. doi: 10.1016/0300-483x(80)90078-5. [DOI] [PubMed] [Google Scholar]
- Reasor M. J. Drug-induced lipidosis and the alveolar macrophage. Toxicology. 1981;20(1):1–33. doi: 10.1016/0300-483x(81)90102-5. [DOI] [PubMed] [Google Scholar]
- Reasor M. J., Heyneman C. A. Disaturated phosphatidylcholine in the pulmonary airspaces of rats treated with chlorphentermine. Biochem Pharmacol. 1983 Mar 1;32(5):939–941. doi: 10.1016/0006-2952(83)90605-6. [DOI] [PubMed] [Google Scholar]
- Reasor M. J., Heyneman C. A., Walker E. R. Chlorcyclizine--induced pulmonary phospholipidosis in rats. Res Commun Chem Pathol Pharmacol. 1982 Nov;38(2):235–246. [PubMed] [Google Scholar]
- Reasor M. J., Koshut R. A., Castranova V. Biochemical characteristics of rat alveolar macrophages with chlorphentermine-induced phospholipidosis: variations with increasing cell size. Exp Mol Pathol. 1979 Oct;31(2):297–307. doi: 10.1016/0014-4800(79)90031-5. [DOI] [PubMed] [Google Scholar]
- Reasor M. J., Koshut R. A., McNulty M. J., Trush M. A. Chemiluminescence from rat alveolar macrophages following induction of phospholipidosis with chlorphentermine. Toxicol Appl Pharmacol. 1980 Mar 15;52(3):497–506. doi: 10.1016/0041-008x(80)90344-0. [DOI] [PubMed] [Google Scholar]
- Reasor M. J., Massey C. A., Koshut R. A., Castranova V. Multinucleation in alveolar macrophages from rats treated with chlorphentermine. Lab Invest. 1982 Feb;46(2):224–230. [PubMed] [Google Scholar]
- Reasor M. J., Trush M. A., Walker E. R. Changes in lysosomal properties of alveolar macrophages of rats treated with chlorphentermine. Toxicol Appl Pharmacol. 1978 Oct;46(1):261–264. doi: 10.1016/0041-008x(78)90157-6. [DOI] [PubMed] [Google Scholar]
- Redding R. A., Douglas W. H., Stein M. Thyroid hormone influence upon lung surfactant metabolism. Science. 1972 Mar 3;175(4025):994–996. doi: 10.1126/science.175.4025.994. [DOI] [PubMed] [Google Scholar]
- Rees S., Constantopoulos G., Barranger J. A., Brady R. O. Organomegaly and histopathology in an animal model of mucopolysaccharidosis induced by suramin. Naunyn Schmiedebergs Arch Pharmacol. 1982 Jun;319(3):262–270. doi: 10.1007/BF00495876. [DOI] [PubMed] [Google Scholar]
- Rhodes M. L. Desquamative interstitial pneumonia. New ultrastructural findings. Am Rev Respir Dis. 1973 Oct;108(4):950–954. doi: 10.1164/arrd.1973.108.4.950. [DOI] [PubMed] [Google Scholar]
- Ridout R. M., Decker R. S., Wildenthal K. Chloroquine-induced lysosomal abnormalities in cultured foetal mouse hearts. J Mol Cell Cardiol. 1978 Feb;10(2):175–183. doi: 10.1016/0022-2828(78)90041-x. [DOI] [PubMed] [Google Scholar]
- Riley J., James J. L., Banaja A. A. The possible role of the frontal and sub-parietal gland systems of the pentastomid Reighardia sternae (Diesing, 1864) in the evasion of the host immune response. Parasitology. 1979 Feb;78(1):53–66. doi: 10.1017/s0031182000048587. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. R., Kolb H., Bergsma D., Huxsoll D., Hopkins J. L. Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci. 1978 Dec;17(12):1158–1175. [PubMed] [Google Scholar]
- Rothman J. E. The molecular basis of mesomorphic phase transitions in phospholipid systems. J Theor Biol. 1973 Jan;38(1):1–16. doi: 10.1016/0022-5193(73)90221-x. [DOI] [PubMed] [Google Scholar]
- Ryrfeldt A. The distribution, elimination, and biotransformation of 14C-cloforex in the mouse and rat. Acta Pharmacol Toxicol (Copenh) 1970;28(5):391–405. doi: 10.1111/j.1600-0773.1970.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Schmien R., Seiler K. U., Wassermann O. Drug-induced phospholipidosis. I. Lipid composition and chlorphentermine content of rat lung tissue and alveolar macrophages after chronic treatment. Naunyn Schmiedebergs Arch Pharmacol. 1974;283(3):331–334. doi: 10.1007/BF00499192. [DOI] [PubMed] [Google Scholar]
- Schutta H. S., Johnson L. Bilirubin encephalopathy in the Gunn rat: a fine structure study of the cerebellar cortex. J Neuropathol Exp Neurol. 1967 Jul;26(3):377–396. doi: 10.1097/00005072-196707000-00003. [DOI] [PubMed] [Google Scholar]
- Schutta H. S., Neville H. E. Effects of cholesterol synthesis inhibitors on the nervous system. A light and electron microscopic study. Lab Invest. 1968 Nov;19(5):487–493. [PubMed] [Google Scholar]
- Seiler K. U., Thiel H. J., Wassermann O. Die Chloroquinkeratopathie als Beispiel einer arzneimittelinduzierten Phospholipidosis (Zugleich ein Beitrag zur Pathogenese der Cornea verticillata) Klin Monbl Augenheilkd. 1977 Jan;170(1):64–73. [PubMed] [Google Scholar]
- Seiler K. U., Wassermann O. Drug-induced phospholipidosis. II. Alterations in the phospholipid pattern of organs from mice, rats and guinea-pigs after chronic treatment with chlorphentermine. Naunyn Schmiedebergs Arch Pharmacol. 1975;288(2-3):261–268. doi: 10.1007/BF00500531. [DOI] [PubMed] [Google Scholar]
- Serabjit-Singh C. J., Wolf C. R., Philpot R. M., Plopper C. G. Cytochrome p-450: localization in rabbit lung. Science. 1980 Mar 28;207(4438):1469–1470. doi: 10.1126/science.6767272. [DOI] [PubMed] [Google Scholar]
- Seydel J. K., Wassermann O. NMR-studies on the molecular basis of drug-induced phospholipidosis--II. Interaction between several amphiphilic drugs and phospholipids. Biochem Pharmacol. 1976 Nov 1;25(21):2357–2364. doi: 10.1016/0006-2952(76)90028-9. [DOI] [PubMed] [Google Scholar]
- Seydel J. K., Wassermann O. NMR-studies on the molecular basis of drug-induced phospholipidosis. Interaction between chlorphentermine and phosphatidylcholine. Naunyn Schmiedebergs Arch Pharmacol. 1973;279(2):207–210. doi: 10.1007/BF00503985. [DOI] [PubMed] [Google Scholar]
- Shikata T., Kanetaka T., Endo Y., Nagashima K. Drug-induced generalized phospholipidosis. Acta Pathol Jpn. 1972 Aug;22(3):517–531. doi: 10.1111/j.1440-1827.1972.tb01849.x. [DOI] [PubMed] [Google Scholar]
- Shinozuka H., Reid I. M., Shull K. H., Liang H., Farber E. Dynamics of liver cell injury and repair. I. Spontaneous reformation of the nucleolus and polyribosomes in the presence of extensive cytoplasmic damage induced by ethionine. Lab Invest. 1970 Sep;23(3):253–267. [PubMed] [Google Scholar]
- Shortland J. R., Darke C. S., Crane W. A. Electron microscopy of desquamative interstitial pneumonia. Thorax. 1969 Mar;24(2):192–208. doi: 10.1136/thx.24.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg D. H., Schutta H. S. The effects of unconjugated bilirubin and related pigments on cultures of rat cerebellum. J Neuropathol Exp Neurol. 1967 Oct;26(4):572–583. doi: 10.1097/00005072-196710000-00005. [DOI] [PubMed] [Google Scholar]
- Smith P., Heath D., Hasleton P. S. Electron microscopy of chlorphentermine lung. Thorax. 1973 Sep;28(5):559–566. doi: 10.1136/thx.28.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P., Heath D., Hasleton P. Effects of prolonged administration of chlorphentermine on the rat lung. Pathol Eur. 1974;9(4):273–287. [PubMed] [Google Scholar]
- Sohal R. S., Peters P. D., Hall T. A. Origin, structure, composition and age-dependence of mineralized dense bodies (concretions) in the midgut epithelium of the adult housefly, Musca domestica. Tissue Cell. 1977;9(1):87–102. doi: 10.1016/0040-8166(77)90051-9. [DOI] [PubMed] [Google Scholar]
- Spangler W. L., Adelman R. D., Conzelman G. M., Jr, Ishizaki G. Gentamicin nephrotoxicity in the dog: sequential light and electron microscopy. Vet Pathol. 1980 Mar;17(2):206–217. doi: 10.1177/030098588001700209. [DOI] [PubMed] [Google Scholar]
- Staiger G. R. Light-microscopic demonstration of drug-induced myelin bodies in the liver of rats. Experientia. 1974 Apr 15;30(4):385–386. doi: 10.1007/BF01921679. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Debuch H. Bis(monoacylglycerin)phosphorsäure--ein Marker-Lipid sekundärer Lysosomen. Hoppe Seylers Z Physiol Chem. 1976 Jun;357(6):803–810. [PubMed] [Google Scholar]
- Stäubli W., Schweizer W., Suter J., Hess R. Ultrastructural and biochemical study of the action of benzoctamine and maprotiline on the rat liver. Agents Actions. 1974 Dec;4(5):391–403. doi: 10.1007/BF01964942. [DOI] [PubMed] [Google Scholar]
- Stäubli W., Schweizer W., Suter J. Some properties of myeloid bodies induced in rat liver by an antidepressant drug (maprotiline). Exp Mol Pathol. 1978 Apr;28(2):177–195. doi: 10.1016/0014-4800(78)90050-3. [DOI] [PubMed] [Google Scholar]
- Sun C. N., White H. J., Lucas G. Crystalloid inclusions in the Schwann cell cytoplasm. Beitr Pathol. 1973 Apr;148(4):402–406. doi: 10.1016/s0005-8165(73)80028-9. [DOI] [PubMed] [Google Scholar]
- Suzuki K., De Paul L. D. Cellular degeneration in developing central nervous system of rats produced by hypocholesteremic drug AY9944. Lab Invest. 1971 Dec;25(6):546–555. [PubMed] [Google Scholar]
- Svendsen O. The effect of phenobarbital on chlorphentermine-induced alveolar foam cells in rats. Toxicol Appl Pharmacol. 1977 Apr;40(1):171–173. doi: 10.1016/0041-008x(77)90128-4. [DOI] [PubMed] [Google Scholar]
- Tashiro Y., Watanabe Y., Enomoto Y. Experimental phospholipidosis induced by 4,4'-diethyl-aminoethoxyhexestrol. Morphological and biochemical interpretations. Acta Pathol Jpn. 1983 Sep;33(5):929–942. [PubMed] [Google Scholar]
- Thelmo W. L., Levine S. Renal lesions induced induced by tilorone and an analog. Ultrastructure and acid phosphatase study. Am J Pathol. 1978 May;91(2):355–360. [PMC free article] [PubMed] [Google Scholar]
- Thoma-Laurie D. L., Walker E. R., Reasor M. J. Chlorphentermine-induced neonatal and maternal pulmonary phospholipidosis in rats. Exp Mol Pathol. 1983 Jun;38(3):310–321. doi: 10.1016/0014-4800(83)90071-0. [DOI] [PubMed] [Google Scholar]
- Thys O., Hildebrand J., Gérin Y., Jacques P. J. Alterations of rat liver lysosomes and smooth endoplasmic reticulum induced by the diazafluoranthen derivative AC-3579. I. Morphologic and biochemical lesions. Lab Invest. 1973 Jan;28(1):70–82. [PubMed] [Google Scholar]
- Tischner K. Effects of chloroquine on neurons of long-term cultures of peripheral and central nervous system. A light and electron microscope study. Acta Neuropathol. 1974;28(3):233–242. doi: 10.1007/BF00719028. [DOI] [PubMed] [Google Scholar]
- Tjiong H. B., Debuch H. Lysosomal bis (monoacylglycero)phosphate of rat liver, its induction by chloroquine and its structure. Hoppe Seylers Z Physiol Chem. 1978 Jan;359(1):71–79. doi: 10.1515/bchm.1978.359.1.71. [DOI] [PubMed] [Google Scholar]
- Tjiong H. B., Lepthin J., Debuch H. Lysosomal phospholipids from rat liver after treatment with different drugs. Hoppe Seylers Z Physiol Chem. 1978 Jan;359(1):63–69. doi: 10.1515/bchm.1978.359.1.63. [DOI] [PubMed] [Google Scholar]
- Tjälve H., Olsson S., Andersson A. The uptake of 14C-chloroquine by mouse pancreatic islets in vitro. Acta Pharmacol Toxicol (Copenh) 1980 Jul;47(1):38–44. doi: 10.1111/j.1600-0773.1980.tb02022.x. [DOI] [PubMed] [Google Scholar]
- Torhorst J., Richter L., Rohr H. P. Entwicklung und Umwandlung lysosomaler Funktionsformen unter besonderer Berücksichtigung des glatten endoplasmatischen Reticulum. Elektronenmikroskopische Untersuchungen am Modell des proximalen Tubulus nach Phenacetinbelastung. Virchows Arch Pathol Anat Physiol Klin Med. 1967;343(1):64–74. [PubMed] [Google Scholar]
- Tousimis A. J., Barron C. N. Chlorpromazine and the eye of the dog. An electron microscopic study. Exp Mol Pathol. 1970 Aug;13(1):89–110. doi: 10.1016/0014-4800(70)90087-0. [DOI] [PubMed] [Google Scholar]
- Trout J. J., Stauber W. T., Schottelius B. A. Increased autophagy in chloroquine-treated tonic and phasic muscles: an alternative view. Tissue Cell. 1981;13(2):393–401. doi: 10.1016/0040-8166(81)90013-6. [DOI] [PubMed] [Google Scholar]
- Tsao S. C., Iga T., Sugiyama Y., Hanano M. Effect of chlorpromazine on isolated rat hepatocytes. Biochem Pharmacol. 1982 Feb 15;31(4):491–497. doi: 10.1016/0006-2952(82)90149-6. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Allan I. J., Newgreen D. F. Extraneuronal effects of 6-hydroxydopamine and extraneuronal uptake of noradrenaline. In-vivo and in-vitro studies on adrenocortical cells of lizards and rats. Cell Tissue Res. 1976 Oct 1;173(1):45–69. doi: 10.1007/BF00219265. [DOI] [PubMed] [Google Scholar]
- Vaughan D. W. Membranous bodies in the cerebral cortex of aging rats: an electron microscope study. J Neuropathol Exp Neurol. 1976 Mar;35(2):152–166. doi: 10.1097/00005072-197603000-00003. [DOI] [PubMed] [Google Scholar]
- Vijeyaratnam G. S., Corrin B. Fine structural alterations in the lungs of iprindole-treated rats. J Pathol. 1974 Dec;114(4):233–240. doi: 10.1002/path.1711140408. [DOI] [PubMed] [Google Scholar]
- Vijeyaratnam G. S., Corrin B. Origin of the pulmonary alveolar macrophage studied in the iprindole-treated rat. J Pathol. 1972 Oct;108(2):115–118. doi: 10.1002/path.1711080204. [DOI] [PubMed] [Google Scholar]
- Vijeyaratnam G. S., Corrin B. Pulmonary alveolar proteinosis developing from desquamative interstitial pneumonia in long term toxicity studies of iprindole in the rat. Virchows Arch A Pathol Pathol Anat. 1973;358(1):1–10. doi: 10.1007/BF00555550. [DOI] [PubMed] [Google Scholar]
- Vinding T., Nielsen N. V. Retinopathy caused by treatment with tamoxifen in low dosage. Acta Ophthalmol (Copenh) 1983 Feb;61(1):45–50. doi: 10.1111/j.1755-3768.1983.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Warhurst D. C., Hockley D. J. Mode of action of chloroquine on Plasmodium berghei and P. cynomolgi. Nature. 1967 May 27;214(5091):935–936. doi: 10.1038/214935a0. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Drug-induced lysosomal changes and nephrotoxicity in rats. Acta Pathol Jpn. 1978 Nov;28(6):867–889. doi: 10.1111/j.1440-1827.1978.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Watts S. D., Atkins A. M. Effects of the schistosomicide 1,7-bis(p-aminophenoxy)heptane (153C51) on lysosomes and membrane stability. Biochem Pharmacol. 1979 Sep 1;28(17):2579–2584. doi: 10.1016/0006-2952(79)90030-3. [DOI] [PubMed] [Google Scholar]
- Watts S. D., Orpin A., MacCormick C. Lysosomes and tegument pathology in the chemotherapy of schistosomiasis with 1,7-bis(p-aminophenoxy)heptane (153C51). Parasitology. 1979 Jun;78(3):287–294. doi: 10.1017/s0031182000051155. [DOI] [PubMed] [Google Scholar]
- Weiss J. N., Ochs A. L., Abedi S., Selhorst J. B. Retinopathy after tilorone hydrochloride. Am J Ophthalmol. 1980 Dec;90(6):846–853. doi: 10.1016/s0002-9394(14)75199-2. [DOI] [PubMed] [Google Scholar]
- Weiss J. N., Weinberg R. S., Regelson W. Keratopathy after oral administration of tilorone hydrochloride. Am J Ophthalmol. 1980 Jan;89(1):46–53. doi: 10.1016/0002-9394(80)90227-5. [DOI] [PubMed] [Google Scholar]
- Wellwood J. M., Simpson P. M., Tighe J. R., Thompson A. E. Evidence of gentamicin nephrotoxicity in patients with renal allografts. Br Med J. 1975 Aug 2;3(5978):278–281. doi: 10.1136/bmj.3.5978.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherrett J. R., Huterer S. Bis-(monoacylglyceryl)-phosphate of rat and human liver: fatty acid composition and NMR spectroscopy. Lipids. 1973 Sep;8(9):531–533. doi: 10.1007/BF02531989. [DOI] [PubMed] [Google Scholar]
- Wherrett J. R., Huterer S. Enrichment of bis-(monoacylglyceryl) phosphate in lysosomes from rat liver. J Biol Chem. 1972 Jul 10;247(13):4114–4120. [PubMed] [Google Scholar]
- Williams P. D., Holohan P. D., Ross C. R. Gentamicin nephrotoxicity. I. Acute biochemical correlates in rats. Toxicol Appl Pharmacol. 1981 Nov;61(2):234–242. doi: 10.1016/0041-008x(81)90413-0. [DOI] [PubMed] [Google Scholar]
- Wilson A. G., Pickett R. D., Eling T. E., Anderson M. W. Studies on the persistence of basic amines in the rabbit lung. Drug Metab Dispos. 1979 Nov-Dec;7(6):420–424. [PubMed] [Google Scholar]
- Wong T. W., Hruban Z. Testicular degeneration and necrosis induced by chlorcyclizine. Lab Invest. 1972 Mar;26(3):278–289. [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Ishibe T., Shinji Y., Kaki-Uchi Y., Seki K. I., Kitani T. Accumulation of acidic phospholipids in a case of hyperlipidemia with hepatosplenomegaly. Lipids. 1970 Jun;5(6):566–571. doi: 10.1007/BF02532747. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Kitani T., Shinji Y., Seki K. Drug-induced lipidosis in human cases and in animal experiments. Accumulation of an acidic glycerophospholipid. J Biochem. 1971 Mar;69(3):613–615. [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Matsuzawa Y., Kitani T., Hiraoka A., Seki K. Studies on drug-induced lipidosis: VII. Effects of bis-beta-diethyl-aminoethylether of hexestrol, chloroquine, homochlorocyclizine, prenylamine, and diazacholesterol on the lipid composition of rat liver and kidney. Lipids. 1976 Aug;11(8):616–622. doi: 10.1007/BF02532875. [DOI] [PubMed] [Google Scholar]
- Yates R. D., Arai K., Rappoport D. A. Fine structure and chemical composition of opaque cytoplasmic bodies of triparanol treated Syrian hamsters. Exp Cell Res. 1967 Sep;47(3):459–478. doi: 10.1016/0014-4827(67)90004-3. [DOI] [PubMed] [Google Scholar]
- Yates R. D., Chen I. L., Mascorro J. A. Some morphological effects of 20,25-diazacholesterol (SC-12937) on adrenocortical cells of the Syrian hamster. Tex Rep Biol Med. 1968 Summer;26(2):241–248. [PubMed] [Google Scholar]
- Zwahlen R., Richardson B. P., Hauser R. E. The production and elimination of myeloid bodies by cultured pancreatic islet cells. J Ultrastruct Res. 1979 Jun;67(3):340–356. doi: 10.1016/s0022-5320(79)80033-7. [DOI] [PubMed] [Google Scholar]
- de la Iglesia F. A., Feuer G., McGuire E. J., Takada A. Morphological and biochemical changes in the liver of various species in experimental phospholipidosis after diethylaminoethoxyhexestrol treatment. Toxicol Appl Pharmacol. 1975 Oct;34(1):28–44. doi: 10.1016/0041-008x(75)90172-6. [DOI] [PubMed] [Google Scholar]