Abstract
Objective
We reported previously that neuronal nitric oxide synthase (nNOS) is the predominant NOS in rat small intestine and is down-regulated by platelet-activating factor (PAF). The severity of the bowel injury induced by PAF is inversely related to its suppressing effect on nNOS. Here, we investigated whether intestinal perfusion is regulated by nNOS and whether tetrahydrobiopterin, a co-factor and stabilizer of nNOS, reverses PAF-induced intestinal hypoperfusion and injury.
Setting
Animal laboratory.
Design
We first examined nNOS regulation of splanchnic blood flow by measuring the perfusion of the heart, lung, ileum, and kidney in rats after a nNOS inhibitor. We then examined the protective effect of tetrahydrobiopterin on PAF-induced bowel injury, mesenteric hypoperfusion, and systemic inflammation.
Subjects
Adult male Sprague-Dawley rats.
Intervention
In part 1 of the experiment, rats were given 7-nitroindazole (a specific nNOS inhibitor, 50 mg·kg−1·day−1). In part 2 of the experiment, rats were treated with tetrahydrobiopterin (20 mg/kg) 5 mins before and 30 mins after PAF challenge (2.2 μg/kg, intravenously)
Measurements
Perfusion of the heart, lung, ileum, and kidney was measured at 1 and 4 days after 7-nitroindazole, using fluorescent microspheres. Intestinal injury and inflammation (myeloperoxidase content), blood perfusion, calcium dependent-NOS activity, and systemic inflammation (hypotension and hematocrit increase) were assessed 1 hr after PAF with and without tetrahydrobiopterin treatment.
Results
In part 1 of the experiment, 7-nitroindazole induced a long-lasting reduction of blood perfusion and inducible NOS expression selectively in the ileum but not in nonsplanchnic organs such as heart, lungs, and kidneys. In part 2, tetrahydrobiopterin protected against PAF-induced intestinal necrosis, hypoperfusion, neutrophil influx, and NOS suppression. It also reversed hypotension and hemoconcentration. Sepiapterin (2 mg/kg, stable tetrahydrobiopterin precursor) also attenuated PAF-induced intestinal injury.
Conclusions
We conclude that nNOS selectively regulates intestinal perfusion. Tetrahydrobiopterin prevents PAF-induced intestinal injury, probably by stabilizing nNOS and maintaining intestinal perfusion.
Keywords: nitric oxide synthase, platelet-activating factor, shock, intestine, intestinal perfusion, tetrahydrobiopterin
Nitric oxide (NO) plays a key role in the homeostatic regulation of the cardiovascular, neuronal, and immune systems (see Alderton et al. (1) for review). NO synthesis is controlled by a family of three NO synthase (NOS) isoforms: the calcium/calmodulin-dependent neuronal NOS (nNOS, type 1), the endothelial NOS (eNOS, type 3), and the calcium-independent, inducible NOS (iNOS, type 2). It is generally accepted that small amounts of NO produced by constitutive NOS (cNOS), including nNOS and eNOS, are important homeostatic regulators of numerous important physiologic functions. In the intestine, NO produced by cNOS plays a pivotal role in maintaining key physiologic functions, such as regulation of intestinal motility (2), prevention of microvascular leakage (3), and maintenance of the integrity of the mucosal barrier (4). In our previous study (5), we identified nNOS as the predominant (>90%) NOS in the intestine, whereas eNOS and iNOS were barely detectable.
PAF (platelet-activating factor), an endogenous phospholipid, is a potent inflammatory mediator that plays a pivotal role in septic shock (6) and intestinal injury induced by lipopolysaccharide (7) or tumor necrosis factor (8). We have established a rat model of ischemic bowel necrosis or necrotizing enterocolitis induced by systemic injection of PAF (9). We found that, in this model, intestinal nNOS (messenger RNA, protein levels, and its activity) was markedly suppressed (5). Furthermore, the severity of the injury was inversely related to the preservation of nNOS activity (10). These observations suggest that PAF induces bowel injury through the down-regulation of intestinal nNOS.
Although presence of nNOS was originally thought to be confined to neural tissue, it is now known to be more widely distributed. Of special interest is its detection in vascular smooth muscle (11). The presence of nNOS in the blood vessel wall suggests a possible regulatory role of this enzyme in blood flow regulation. Our previous study (5) showed that, unlike the heart, the small intestine contains very little eNOS, and nNOS is the predominant NOS. Thus, we hypothesize that: 1) the regulation of the blood flow and perfusion in the intestine is controlled by nNOS; 2) that this phenomenon may be specific for the gastrointestinal tract because eNOS, the putative regulator of vascular homeostasis, is present in abundant amounts in heart and lung; and 3) that PAF reduces intestinal blood flow by suppressing nNOS.
Tetrahydrobiopterin (BH4) is an essential cofactor for the activity of all three NOS isoenzymes (1). BH4 may stabilize the nNOS dimers (12), inhibit nNOS monomerization (13), prevent/reverse the NO-induced inhibition of nNOS (14), and stabilize its quaternary structure (15). Recent studies have shown that BH4 and its precursor, sepiapterin, reduced the ischemia/reperfusion injury of the stomach (16). We thus hypothesize that administration of BH4 or sepiapterin decreases nNOS degradation, reverses PAF-induced splanchnic hypoperfusion, and prevents PAF-induced bowel necrosis and circulatory failure.
METHODS
Blood Perfusion Rate Change Induced by 7-Nitroindazole in the Small Intestine, Heart, Lung, and Kidney
Five groups of young Sprague-Dawley rats (150–200 g) were treated as follows: A) 7-nitroindazole (7-NI, a specific nNOS inhibitor (17); CalBiochem, San Diego, CA; 100 mg/mL, in dimethyl sulfoxide), 50 mg·kg−1·day−1, intraperitoneally, for 1 day; B) 7-NI (50 mg·kg−1·day−1, intraperitoneally) for four consecutive days; C) vehicle for 1 day; D) vehicle for four consecutive days; and E) no treatment (baseline controls). The animals in groups A–D were subjected to organ blood perfusion measurement at the end of the first or fourth day of the treatments. Group E was measured at day 0.
The blood perfusion rate in various organs was measured using the fluorescent microsphere method as described in “Manual for Using the Fluorescent Microsphere to Measure the Regional Organ Perfusion” (18) and elsewhere (19, 20). Briefly, the rats were anesthetized with Nembutal (65 mg/kg, intraperitoneally), tracheotomized, and catheterized via the carotid artery, jugular vein, and femoral artery; 0.2 mL heparin (1000 units/mL, intravenously) was injected before the experiment. A suspension of fluorescent microspheres (0.01 mm [Molecular Probe, Eugene, OR], 1.0 × 106/mL in PBS containing 5% dextran and 0.025% Tween-80, total volume of 0.15 mL) was injected into the aorta through the carotid artery catheter in 10 secs. At the time of microsphere injection, a “reference” blood sample was drawn into a heparinized syringe through the femoral artery catheter at the rate of 0.39 mL/min via a syringe pump (Harvard Apparatus, Holliston, MA). One minute after the injection, the pump was stopped. The animal's blood circulation was also stopped immediately by a quick injection of a lethal dose of Nembutal through the venous catheter. The major organs, including heart, lung, both kidneys, ileum, colon, and liver, depending on the experiment, were collected, blotted dry, and weighed. The tissue samples and the reference blood samples were digested with 4 N potassium hydroxide. The microspheres trapped in the samples were retrieved by filtration and dissolved in 2-ethoxyethyl acetate. Fluorescence intensity of the solution was determined using a Perkin-Elmer LS-55 fluorescent spectrophotometer (excitation wavelength, 535 nm; emission, 555 nm). The regional perfusion rate (representing a “snapshot” at the time of blood collection) of the organ was calculated as: Perfusion rate = [(FItissue/FIblood) × 0.39 mL/min]/Wtissue, where FI is fluorescent intensity and W is wet tissue weight. The perfusion rate of both kidneys was compared and found to be significantly correlated (r2 = .9256, p < .05), indicating adequate mixing of the microsphere in vivo. To examine the possible error resulted from tissue edema, parallel experiments were done and wet/dry weight ratio was determined. We found no significant difference among the mean values of such ratios in the three groups (p > .05).
To quantify tissue iNOS protein content, intestinal or lung tissue was homogenized and the protein concentration determined as described previously (10). After precleaning with protein-A agarose, 1.0 mL of tissue lysate (0.25 mg of protein) was treated with 30 μL of anti-NOS II Ab M-19 (Santa Cruz Biotechnology, Santa Cruz, CA), followed by protein A. The bound immune complex was eluted by Laemmli buffer, boiled, and the supernatant loaded on a 7.5% sodium dodecyl sulfatepolyacrylamide gel for electrophoresis resolution. The resolved protein was detected by Western blot using anti-NOS II Ab M-19 and electrogenerated chemiluminescence system (10).
Protective Effect of BH4 and Sepiapterin on PAF-Induced Ischemic Bowel Injury
Young male Sprague-Dawley rats (120–150 g) were anesthetized with Nembutal, tracheotomized, and catheterized via carotid artery and jugular vein for continuous blood pressure recording, blood sampling, and drug administration. The animals were divided into four groups and treated as follows. A) PAF (1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocho-line, 2.2 μg/kg, intravenously; Sigma, St. Louis, MO) was injected at time 0, and saline was injected (intravenously) 5 mins before and 30 mins after PAF. B) BH4 (Sigma), 40 mg/kg, divided into two doses, was injected intravenously 5 mins before and 30 mins after PAF (injected at time 0) + PAF. C) For the control, saline (vehicle) was injected intravenously at −5, 0, and 30 mins. D) Sepiapterin (2 mg/kg, injected intravenously 2 hrs before PAF) + PAF. Each experiment included at least one rat receiving saline (C) and one receiving PAF (A). The experiment was terminated 60 mins after PAF injection. Mean arterial pressure was continuously recorded. Blood samples were collected at the beginning and the end of the experiment for the measurement of hematocrit.
At 60 mins after PAF administration, the animals were killed. The small intestine was removed and representative sections were taken, fixed in formalin, embedded in paraffin, and hematoxylin and eosin sections were examined microscopically (21). The remainder was minced with scissors, snap-frozen in liquid nitrogen, and stored in a −80°C freezer for further assays. The severity of the gross and microscopic tissue injury was evaluated using a scoring system as reported previously (21). In a separate set of experiments, the effect of PAF administration on the organ perfusion rate at 60 mins and the protective effect of BH4 were also determined as described above.
The activity of intestinal cNOS (>90% attributed to nNOS) was determined by calcium-dependent [14C]l-arginine conversion, as previously published (10). Briefly, homogenized intestinal tissue was spun at 20,000 × g at 4°C for 20 mins. Two reaction systems were prepared for each sample in a phosphate buffer (pH 7.2) containing 0.4 mM CaCl2, 2 mM MgCl2, 2 μM flavin adenine dinucleotide, 1 μM flavin adenine mononucleotide, 6 μM BH4, and 1 mM [14C]l-arginine. System 2 also contained EDTA (2.5 mM). The reaction was initiated by adding 10 μL of 10 mM NADPH. After incubating at 37°C, stopping buffer was added. The sample was then passed through a Dowex-50W cation exchange column and eluted. The activity of cNOS is defined as the difference of activities between systems 1 and 2.
Myeloperoxidase (a marker enzyme for neutrophils) assay was used to detect polymorphonuclear neutrophil influx into the intestine, as previously described (21). Briefly, homogenized intestinal tissue (in 0.05 M potassium phosphate buffer containing 0.5% hexadecyltrimethyl-ammonium bromide and 5 mM EDTA) was sonicated, reacted with substrate (O-dianisidine HCl + H2O2 in potassium phosphate buffer), and the optical density read at 460 nm.
All animal experiments were approved by the Institutional Animal Care and Use Committee, and the care and handling of the animals were in accordance with National Institutes of Health guidelines.
Statistical Analysis
One-way analysis of variance was employed to analyze the data of multiple groups, and two-sided Student's t-test was used for the comparison of any two single groups because all data were conformed following normal distribution. Data are presented as mean ± sem; .05 was used as the level of significance for each experiment. Bonferroni correction was used for the comparison of multiple groups in analysis of variance.
RESULTS
Suppression of nNOS by 7-NI
Suppression of nNOS by 7-NI selectively reduces blood perfusion rate in the small intestine (but not heart, lung or kidney), and 7-NI–induced iNOS expression is also intestine-specific. We compared the regional blood perfusion rate in the ileum of the rats treated either with saline or 7-NI, a specific nNOS inhibitor (17), for 1 or 4 days. We found (Fig. 1A) that the ileal regional blood perfusion rate in 7-NI–treated animals became significantly lower in comparison with the sham-treated animals 1 day after the beginning of 7-NI administration and stayed so for the duration of 7-NI administration.
Figure 1.
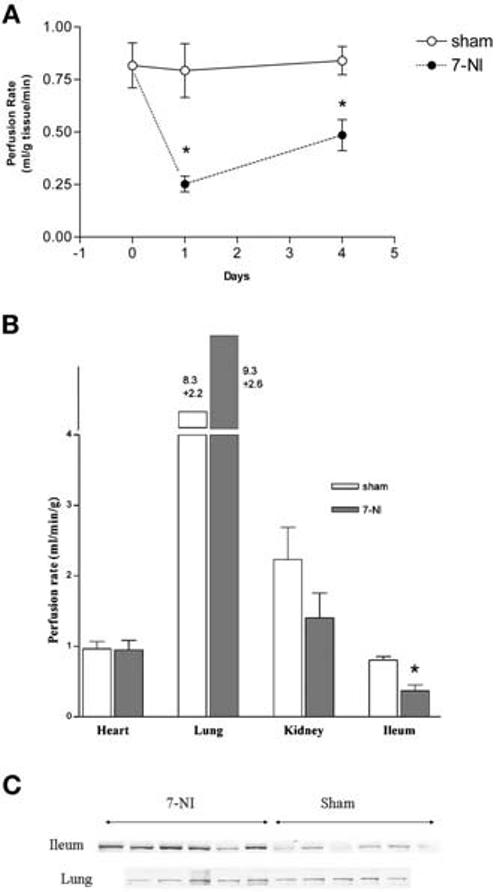
Effect of 7-nitroindazol (7-NI) on organ perfusion and inducible nitric oxide synthase (iNOS) expression. A, time course of small-intestine blood perfusion in response to 7-NI (50 mg·kg−1·day−1, intraperitoneally, for one or four consecutive days). Sham control, vehicle for one or four consecutive days. Each point represents mean ± sem of four animals; *significantly different from sham group, p < .05. B, blood perfusion of heart, lung, kidney, and ileum 24 hrs after 7-NI (50 mg·kg−1·day−1, intraperitoneally) and sham control. (n = 12 for sham; n = 9 for 7-NI). Data displayed as mean ± sem; *significantly different from sham group. p < .05. C, Western blot showing iNOS content in ileum and lung in 7-NI–treated (50 mg·kg−1·day−1, intraperitoneally for 1 day) and sham-treated (vehicle for 1 day) rats. iNOS protein was immunoprecipitated using an anti-NOS II antibody M-19 (Santa Cruz Biotechnology, Santa Cruz, CA), incubated with protein-A agarose beads (Santa Cruz Bio-technology), and eluted before Western blotting. Each lane represents one animal, n = 6 in each group (sham, 7-NI) for ileum, and n = 5 in each group for lung.
To further confirm the finding that nNOS (instead of eNOS) plays a major role in the regulation of blood circulation in the small intestine, we compared the effect of 7-NI administration on the regional blood perfusion in heart, lung, kidney, and ileum at 24 hrs (the time point of 24 hrs was selected because the intestinal flow reached its lowest point at this time). It was found, as shown in Figure 1B, that among all the four organs studied, the small intestine is the only organ where 7-NI induced significant reduction of regional blood perfusion. This finding suggests that the blood flow in the intestine, unlike that in other organs, is regulated by nNOS, the predominant NOS found in the intestine. Our previous study showed that inhibition of intestinal nNOS results in nuclear factor-κB activation and subsequent iNOS induction (22), suggesting a gene-regulating role of intestinal nNOS. To investigate whether this nNOS effect is also selective for intestine, we examined the iNOS tissue content, before and after 7-NI treatment, in ileum and lung. As shown in Fig. 1C, 7-NI treatment increased iNOS expression in the ileum but not in the lung, in which eNOS is the predominant cNOS.
PAF Down-regulates Intestinal nNOS and BH4 Reverses PAF-Induced Inhibition of Intestinal nNOS
To investigate the protective effect of BH4, an essential cofactor of NOS, on nNOS, we examined the intestinal cNOS activity (>90% of this activity is accounted for by nNOS (5)) in BH4-treated animals challenged with PAF. Due to the short half-life of BH4 in vivo, it was given 5 mins before and 30 mins after PAF challenge. As shown in Fig. 2, PAF markedly suppressed cNOS activity, which was almost completely prevented by BH4 treatment.
Figure 2.
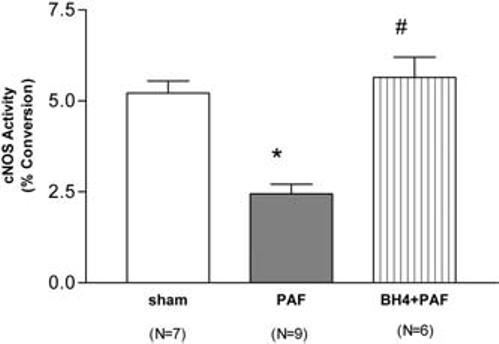
Intestinal constitutive nitric oxide synthase (cNOS) activity (>90% is accounted for by neuronal NOS) in rats receiving vehicle (sham), platelet-activating factor (PAF, 2.2 μg/kg), and tetrahydrobiopterin (BH4, 40 mg/kg, given in two doses, 5 mins before and 30 mins after PAF) + PAF. *Significantly different from sham group (p < .05); #significantly different from PAF group (p < .05).
Pretreatment with BH4
Pretreatment with BH4 protects against PAF-induced circulatory shock, hemoconcentration, and splanchnic blood flow reduction; recovers intestinal perfusion; and protects the small intestine against PAF-induced polymorphonuclear neutrophil sequestration and tissue injury. Sepiapterin, a stable precursor of BH4, also ameliorates PAF-induced injury. If our hypothesis that nNOS regulates intestinal blood flow and PAF suppresses nNOS is true, it is logical to infer that 1) PAF reduces small intestinal perfusion because mesenteric flow decreases after PAF (23) and that 2) BH4, which protects nNOS against PAF action, reverses PAF-induced intestinal hypoperfusion. As shown in Figure 3A, 60 mins after PAF administration, the small intestinal perfusion rate dropped to <40% of control value in sham-operated animals. BH4 treatment completely reversed PAF-induced intestinal hypoperfusion. The effect of PAF on blood flow and the protection of BH4 were also observed in other splanchnic organs. The hepatic blood flow dropped to almost 50% of control, which was also reversed by BH4 treatment (data not shown). Interestingly, the decreased colonic blood flow was only partially protected by BH4 treatment (data not shown).
Figure 3.
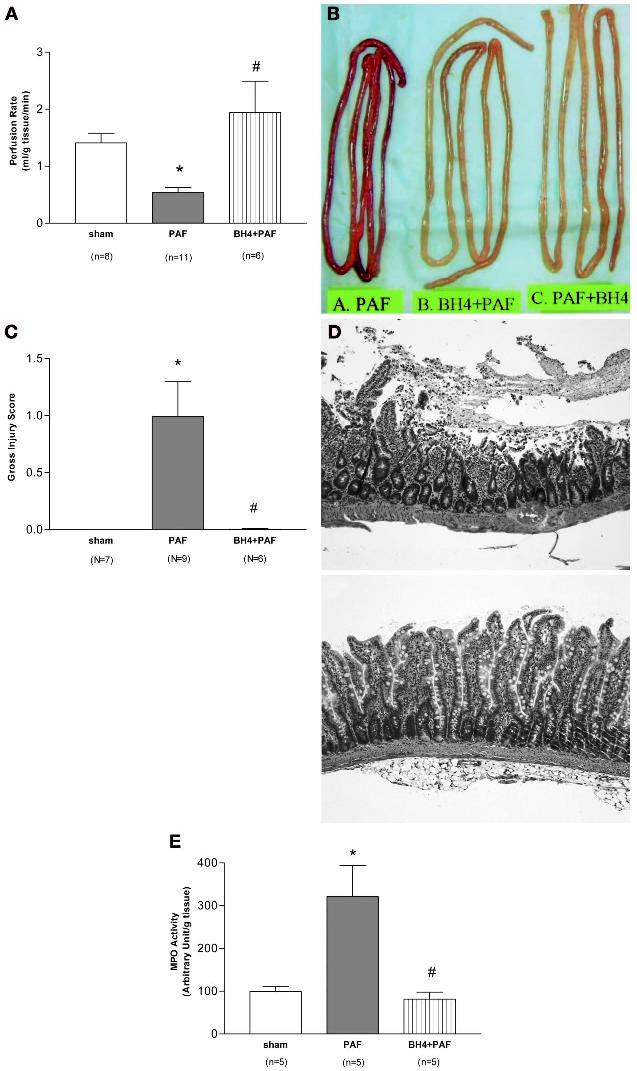
Tetrahydrobiopterin (BH4) reverses platelet-activating factor (PAF)-induced reduction in small-intestine perfusion and protects against PAF-induced intestinal injury. A, small-intestine perfusion rate in rats receiving vehicle (sham), PAF (2.2 μg/kg, intravenously), and BH4 (20 mg/kg at 5 mins before and 20 mg/kg at 30 mins after PAF) + PAF (mean ± sem). *Significantly different from sham group (p < .05); #significantly different from PAF group (p < .05). B, gross appearance of the small intestine from rats treated with PAF (2.2 μg/kg) and BH4 (40 mg/kg, divided into two doses, 5 mins before and 30 mins after PAF) + PAF. A representative animal is shown. An additional animal with posttreatment of BH4 (i.e., BH4, 40 mg/kg, divided into two doses, administered 20 and 50 mins given after PAF) is also shown. C, gross intestinal injury score in rats treated with vehicle (sham control), PAF (2.2 μg/kg), and BH4 (40 mg/kg, divided into two doses, 5 mins before and 30 mins after PAF) + PAF. Data provided as mean ± sem; *significantly different from sham group (p < .05); #significantly different from PAF group (p < .05). D, histologic appearance of small intestine from rats receiving PAF (2.2 μg/kg, top panel) and BH4 (40 mg/kg, divided in two doses) + PAF (2.2 μg/kg, bottom panel). E, small-intestine myeloperoxidase (MPO) activity in rats receiving vehicle (sham control), PAF (2.2 μg/kg,), BH4 (40 mg/kg, divided in two doses) + PAF. Data provided as mean ± sem; *significantly different from sham group (p < .05); #significantly different from PAF group (p < .05).
PAF (group A), at the dose used, induced moderate small-intestine injury (Fig. 3, B–D). Pretreatment with BH4 protected against PAF-induced gross intestinal injury (Fig. 3, B and C). The protection was corroborated by microscopic examination (Fig. 3D). Animals pretreated with BH4 (group B) showed very mild intestinal injury. The microscopic injury score was 0.04 ± 0.02, significantly lower in comparison with the injury score of the PAF group (1.10 ± 0.29, p < .05). Sham control groups showed no injury. BH4 treatment (group B) also significantly prevented the PAF-induced neutrophil sequestration (reflected by the increase of the intestinal myeloperoxidase, a marker enzyme of neutrophils) in the small intestine (Fig. 3E).
We also examined the protective effect of sepiapterin, a precursor for BH4 (which presumably increases endogenous BH4 synthesis) against PAF-induced bowel injury. As shown in Figure 4, sepiapterin (2 mg/kg, intraperitoneally), given 2 hrs before PAF challenge, significantly reduced PAF-induced gross bowel injury. Sepiapterin also significantly reduced PAF-induced hypotension and hemoconcentration. However, histologic examination revealed that the protection was only partial; the histologic score was significantly less than the PAF group but higher than the BH4 group.
Figure 4.
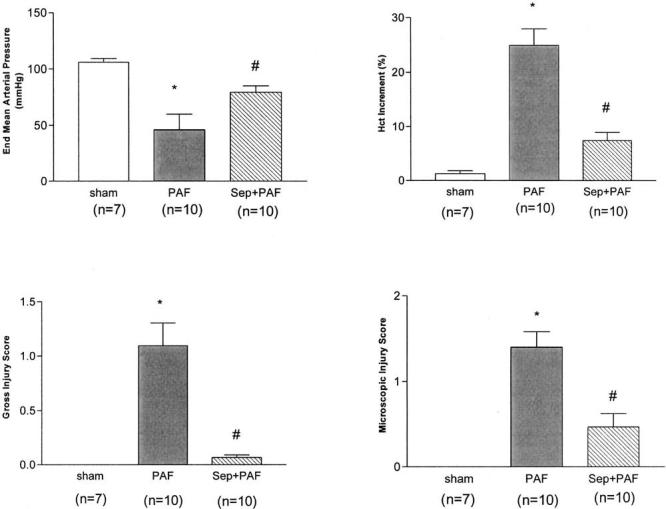
Protective effect of sepiapterin (Sep) on platelet-activating factor (PAF)-induced hypotension, hemoconcentration, and bowel injury. Sepiapterin (2 mg/kg, intravenously) was administered 2 hrs before PAF (2.2 μg/kg, intravenously). End mean arterial pressure was measured 60 mins after PAF administration. Hematocrit increment = (hematocrit60 mins − hematocrit0 min). Data provided as mean ± sem; *significantly different from sham group (p < .05); #significantly different from PAF group (p < .05).
BH4 pretreatment significantly alleviated PAF-induced shock (defined as end mean arterial pressure of <60 mm Hg, p < .05). The mean arterial pressure at the end of the experiments of each group was: sham (n = 7), 106.0 ± 3.3; PAF (n = 9), 55.9 ± 10.4; and BH4+PAF (n = 6), 86.7 ± 5.58. It also ameliorated PAF-induced hemoconcentration (an index for increased permeability, p < .05). The baseline hematocrit of each group was: sham, 41.0% ± 0.4%; PAF, 39.3% ± 0.5%; BH4+PAF, 36.1% ± 2.5%. The increment of hematocrit at the end of the experiment was: sham, 1.3% ± 0.6%; PAF, 14.6% ± 2.6%; BH4+PAF, 6.9% ± 1.0%.
DISCUSSION
Among the three NOS isoforms (1), nNOS was originally thought to be present mainly in neuronal tissue. Recent evidence shows that nNOS is also detected in other types of cells and tissues (24–27), including endothelial cells (28) and vascular smooth muscle (11, 27). It is abundant in human (29) and rat (30) intestine. We have shown that nNOS is the predominant NOS isoform in the intestine (>90% of NOS) (5). Besides regulating intestinal motility (2), intestinal nNOS may also participate in the regulation of iNOS via nuclear factor-κB (22). Whether nNOS has any function in controlling mesenteric perfusion remains unclear. In two vascular injury models, nNOS, absent before injury, became prominently expressed in the vascular neointima and smooth muscle and prevented the vasoconstriction response to stimuli (31). However, it is unclear whether nNOS has any physiologic function in regulating splanchnic blood flow in normal animals. Because the intestine, unlike other organs, contains very little eNOS (5), it is possible that intestinal nNOS functions as eNOS to regulate blood perfusion. Our study is the first to demonstrate selective regulation of splanchnic blood flow by nNOS, as 7-NI reduces blood flow to the intestine but not the heart, lung, or kidney. (We also did experiments injecting microspheres to the lung via the jugular vein and compared pulmonary blood flow in control and 7-NI–treated rats [n = 4 in each] and found that 7-NI did not cause reduction of the pulmonary blood flow [data not shown].)
BH4 is an essential cofactor for the activity of all three NOS isoenzymes (1). Sepiapterin is a precursor of BH4. The effects of BH4 on nNOS include inhibiting nNOS monomerization (13), stabilizing the nNOS dimers, and reducing their susceptibility to phosphorylation by protein kinase C (12), allosterically activating nNOS and stabilizing quaternary structure (15), and inhibiting superoxide generation by nNOS (32, 33), which inactivates nNOS itself. BH4 improves endothelial function in vivo (34, 35), and BH4 and its precursor, sepiapterin, reduce the ischemia/reperfusion injury of the stomach (16). Treatment of mice with oral BH4 increases NO, reduces vascular reactive oxygen species (ROS) production, and blunts the increase in blood pressure due to deoxycorticosterone acetate–salt hypertension (36). PAF may be important in mediating bowel injury because a) the intestine is a major site of PAF production in endotoxemia (37), and the PAF receptor is expressed in highest quantity in this organ (38); b) systemic administration of PAF causes intestinal necrosis (9); and c) PAF antagonists prevent endotoxin-induced intestinal injury (7). Besides causing mesenteric vasoconstriction (39) and reducing mesenteric blood flow (23), PAF also suppresses the protective nNOS activity (5, 10) and reduces intestinal content of nNOS messenger RNA and protein (5). Furthermore, the severity of the intestinal injury is inversely proportional to its nNOS activity (10). These observations suggest that PAF induces bowel injury by down-regulating its nNOS and reducing intestinal perfusion.
It is unclear how PAF causes degradation of nNOS messenger RNA and protein. It is possible that PAF suppresses nNOS partly via ROS. It is well known that PAF induces ROS production from eosinophils, polymorphonuclear neutrophils, and monocytes (40) in vitro. Furthermore, we have shown that PAF markedly induces the conversion of xanthine dehydrogenase to xanthine oxidase in the intestinal epithelium (41) and the subsequent massive ROS production in vivo because PAF-induced injury is largely prevented by allopurinol (23, 41) and antioxidants (23). We have also observed a significant reduction of intestinal nNOS activity after ischemia/reperfusion (unpublished data). BH4 is especially vulnerable to ROS, as shown by in vitro demonstration that oxygen free radical causes a modest (and peroxynitrite a striking) decay of BH4 over 500 secs at pH 7.4 (42). Furthermore, nNOS (43) can produce ROS; this response is enhanced when the enzyme is “uncoupled” in the absence of BH4 (36). Simultaneous release of NO and ROS when BH4 is low may lead to the production of highly toxic radicals. Thus, an increase in cellular BH4 not only obviates NOS dysfunction but also corrects the balance between ROS/NO production (32, 43). The present study is the first to show that BH4 prevents PAF-induced bowel injury, systemic inflammation, and shock, and one possible mechanism may be via protection of intestinal nNOS and consequent preservation of the intestinal perfusion. We have also examined whether BH4 given after PAF (at 20 and 50 mins) could reverse the injurious effect of PAF. Animals in this group (n = 3) showed less bowel injury (microscopic score, 0.08 ± 0.03) and higher intestinal cNOS activity (4.653% ± 1.432% substrate conversion) in comparison with the PAF-treated group. The protective effect of sepiapterin, the precursor of BH4 (reviewed in Thony et al. (44)) was less than BH4. This might be due to limited drug delivery into tissue due to its poor solubility.
Neuronal nitric oxide synthase selectively regulates intestinal perfusion, and tetrahydrobiopterin prevents platelet-activating factor–induced intestinal injury, probably by stabilizing neuronal nitric oxide synthase and maintaining intestinal perfusion.
BH4 is a physiologic substance, inexpensive, and nontoxic and thus has potential therapeutic use. However, it has a very short half-life in vivo. Development of stable, soluble analogues of BH4 may have therapeutic potential for the prevention or treatment of devastating diseases associated with bowel necrosis, such as septic shock, ischemic bowel necrosis, and neonatal necrotizing enterocolitis.
ACKNOWLEDGMENTS
We thank Xueli Liu and Jin Chen for excellent technical assistance and Dr. Roopa Seshadri for help with the statistical analysis of the data.
Footnotes
Supported, in part, by National Institutes of Health grants RO1-DK34574 and RO1-HD042581.
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: Structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421–430. doi: 10.1007/s00535-003-1094-y. [DOI] [PubMed] [Google Scholar]
- 3.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 4.Kanwar S, Wallace JL, Befus D, et al. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994;266:G222–G229. doi: 10.1152/ajpgi.1994.266.2.G222. [DOI] [PubMed] [Google Scholar]
- 5.Qu XW, Wang H, Rozenfeld RA, et al. Type I nitric oxide synthase (NOS) is the predominant NOS in rat small intestine: Regulation by platelet-activating factor. Biochim Biophys Acta. 1999;1451:211–217. doi: 10.1016/s0167-4889(99)00076-2. [DOI] [PubMed] [Google Scholar]
- 6.Handley DA. Platelet-activating factor as a mediator of endotoxin-related diseases. In: Handley DA, Saunders RN, Houlihan WJ, et al., editors. Platelet Activating Factor in Endotoxin and Immune Diseases. Marcel Dekker; New York: 1990. [Google Scholar]
- 7.Hsueh W, Gonzalez Crussi F, Arroyave JL. Platelet-activating factor: An endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987;1:403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988;81:1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Crussi F, Hsueh W. Experimental model of ischemic bowel necrosis: The role of platelet-activating factor and endotoxin. Am J Pathol. 1983;112:127–135. [PMC free article] [PubMed] [Google Scholar]
- 10.Qu XW, Rozenfeld RA, Huang W, et al. Roles of nitric oxide synthases in platelet-activating factor–induced intestinal necrosis in rats. Crit Care Med. 1999;27:356–364. doi: 10.1097/00003246-199902000-00043. [DOI] [PubMed] [Google Scholar]
- 11.Buchwalow IB, Podzuweit T, Bocker W, et al. Vascular smooth muscle and nitric oxide synthase. FASEB J. 2002;16:500–508. doi: 10.1096/fj.01-0842com. [DOI] [PubMed] [Google Scholar]
- 12.Okada D. Tetrahydrobiopterin-dependent stabilization of neuronal nitric oxide synthase dimer reduces susceptibility to phosphorylation by protein kinase C in vitro. FEBS Lett. 1998;434:261–264. doi: 10.1016/s0014-5793(98)00993-4. [DOI] [PubMed] [Google Scholar]
- 13.Reif A, Frohlich LG, Kotsonis P, et al. Tetrahydrobiopterin inhibits monomerization and is consumed during catalysis in neuronal NO synthase. J Biol Chem. 1999;274:24921–24929. doi: 10.1074/jbc.274.35.24921. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi T, Sagami I, Daff S, et al. Important role of tetrahydrobiopterin in no complex formation and interdomain electron transfer in neuronal nitric-oxide synthase. Biochem Biophys Res Commun. 2001;282:1092–1097. doi: 10.1006/bbrc.2001.4697. [DOI] [PubMed] [Google Scholar]
- 15.Kotsonis P, Frohlich LG, Shutenko ZV, et al. Allosteric regulation of neuronal nitric oxide synthase by tetrahydrobiopterin and suppression of auto-damaging superoxide. Biochem J. 2000;346(Pt 3):767–776. [PMC free article] [PubMed] [Google Scholar]
- 16.Ishii M, Shimizu S, Nawata S, et al. Involvement of reactive oxygen species and nitric oxide in gastric ischemia-reperfusion injury in rats: Protective effect of tetrahydrobiopterin. Dig Dis Sci. 2000;45:93–98. doi: 10.1023/a:1005413511320. [DOI] [PubMed] [Google Scholar]
- 17.Schulz JB, Matthews RT, Muqit MM, et al. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- 18.Division of Pulmonary and Critical Medicine. Fluorescent Microsphere Resource Center, University of Washington; Seattle: 1999. Manual for Using the Fluorescent Microsphere to Measure the Regional Organ Perfusion. [Google Scholar]
- 19.Tuma RF, Vasthare US, Irion GL, et al. Considerations in use of microspheres for flow measurement in anesthetized rat. Am J Physiol. 1986;250:H137–H143. doi: 10.1152/ajpheart.1986.250.1.H137. [DOI] [PubMed] [Google Scholar]
- 20.Thaete LG, Dewey ER, Neerhof MG. Endothelin and regulation of uterine and placental perfusion in hypoxia-induced fatal growth restriction. J Soc Gynecol Invest. 2004;11:14–21. doi: 10.1016/j.jsgi.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Qu XW, Huang W, et al. Role of leukocyte β2-integrin in PAF-induced shock and intestinal injury. Am J Physiol. 1996;270:G184–G190. doi: 10.1152/ajpgi.1996.270.1.G184. [DOI] [PubMed] [Google Scholar]
- 22.Qu XW, Wang H, De Plaen IG, et al. Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B. FASEB J. 2001;15:439–446. doi: 10.1096/fj.99-0343com. [DOI] [PubMed] [Google Scholar]
- 23.Cueva JP, Hsueh W. Role of oxygen derived free radicals in platelet activating factor induced bowel necrosis. Gut. 1988;29:1207–1212. doi: 10.1136/gut.29.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 25.Barouch LA, Harrison RW, Skaf MW, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase iso-forms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 26.Premaratne S, Xue C, McCarty JM, et al. Neuronal nitric oxide synthase: Expression in rat parietal cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G308–G313. doi: 10.1152/ajpgi.2001.280.2.G308. [DOI] [PubMed] [Google Scholar]
- 27.Ermert M, Ruppert C, Gunther A, et al. Cell-specific nitric oxide synthase-isoenzyme expression and regulation in response to endotoxin in intact rat lungs. Lab Invest. 2002;82:425–441. doi: 10.1038/labinvest.3780436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenblum WI, Murata S. Antisense evidence for two functionally active forms of nitric oxide synthase in brain microvascular endothelium. Biochem Biophys Res Commun. 1996;224:535–543. doi: 10.1006/bbrc.1996.1061. [DOI] [PubMed] [Google Scholar]
- 29.Saur D, Paehge H, Schusdziarra V, et al. Distinct expression of splice variants of neuronal nitric oxide synthase in the human gastrointestinal tract. Gastroenterology. 2000;118:849–858. doi: 10.1016/s0016-5085(00)70171-5. [DOI] [PubMed] [Google Scholar]
- 30.Huber A, Saur D, Kurjak M, et al. Characterization and splice variants of neuronal nitric oxide synthase in rat small intestine. Am J Physiol. 1998;275:G1146–G1156. doi: 10.1152/ajpgi.1998.275.5.G1146. [DOI] [PubMed] [Google Scholar]
- 31.Morishita T, Tsutsui M, Shimokawa H, et al. Vasculoprotective roles of neuronal nitric oxide synthase. FASEB J. 2002;16:1994–1996. doi: 10.1096/fj.02-0155fje. [DOI] [PubMed] [Google Scholar]
- 32.Vasquez-Vivar J, Hogg N, Martasek P, et al. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 33.Rosen GM, Tsai P, Weaver J, et al. The role of tetrahydrobiopterin in the regulation of neuronal nitric-oxide synthase-generated superoxide. J Biol Chem. 2002;277:40275–40280. doi: 10.1074/jbc.M200853200. [DOI] [PubMed] [Google Scholar]
- 34.Katusic ZS. Vascular endothelial dysfunction: Does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol. 2001;281:H981–H986. doi: 10.1152/ajpheart.2001.281.3.H981. [DOI] [PubMed] [Google Scholar]
- 35.Sun H, Patel KP, Mayhan WG. Tetrahydrobiopterin, a cofactor for NOS, improves endothelial dysfunction during chronic alcohol consumption. Am J Physiol Heart Circ Physiol. 2001;281:H1863–H1869. doi: 10.1152/ajpheart.2001.281.5.H1863. [DOI] [PubMed] [Google Scholar]
- 36.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu X, Huang L, Burthart T, et al. Endotoxin induces PAF production in the rat ileum: Quantitation of tissue PAF by an improved method. Prostaglandins. 1996;51:249–262. doi: 10.1016/0090-6980(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Tan X, Chang H, et al. Regulation of platelet-activating factor receptor gene expression in vivo by endotoxin, platelet-activating factor and endogenous tumour necrosis factor. Biochem J. 1997;322:603–608. doi: 10.1042/bj3220603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Hsueh W. PAF-induced bowel necrosis: Effects of vasodilators. Dig Dis Sci. 1991;36:634–640. doi: 10.1007/BF01297031. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman GA, McIntyre TM, Prescott SM, et al. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30:S294–S301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 41.Qu XW, Rozenfeld RA, Huang W, et al. The role of xanthine oxidase in platelet-activating factor induced intestinal injury in the rat. Gut. 1999;44:203–211. doi: 10.1136/gut.44.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laursen JB, Somers M, Kurz S, et al. Endothelial regulation of vasomotion in apoEdeficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 43.Xia Y, Dawson VL, Dawson TM, et al. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Nat Acad Sci U S A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]


