Abstract
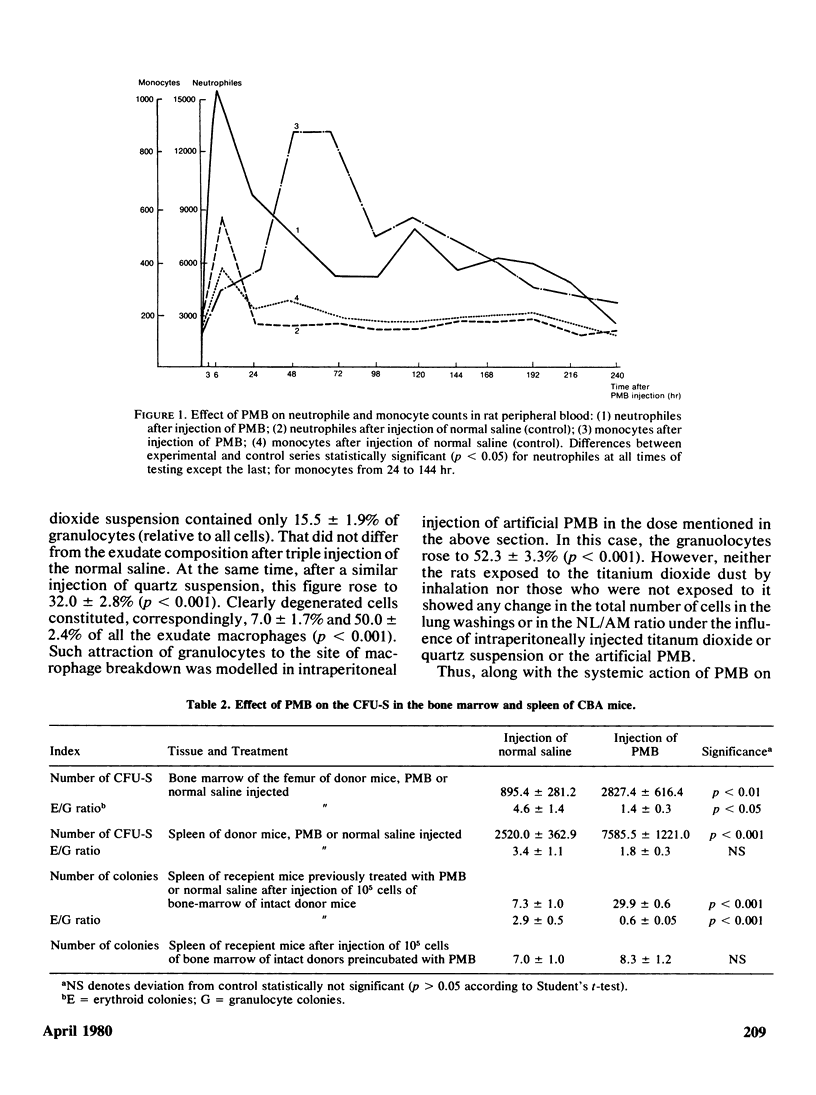
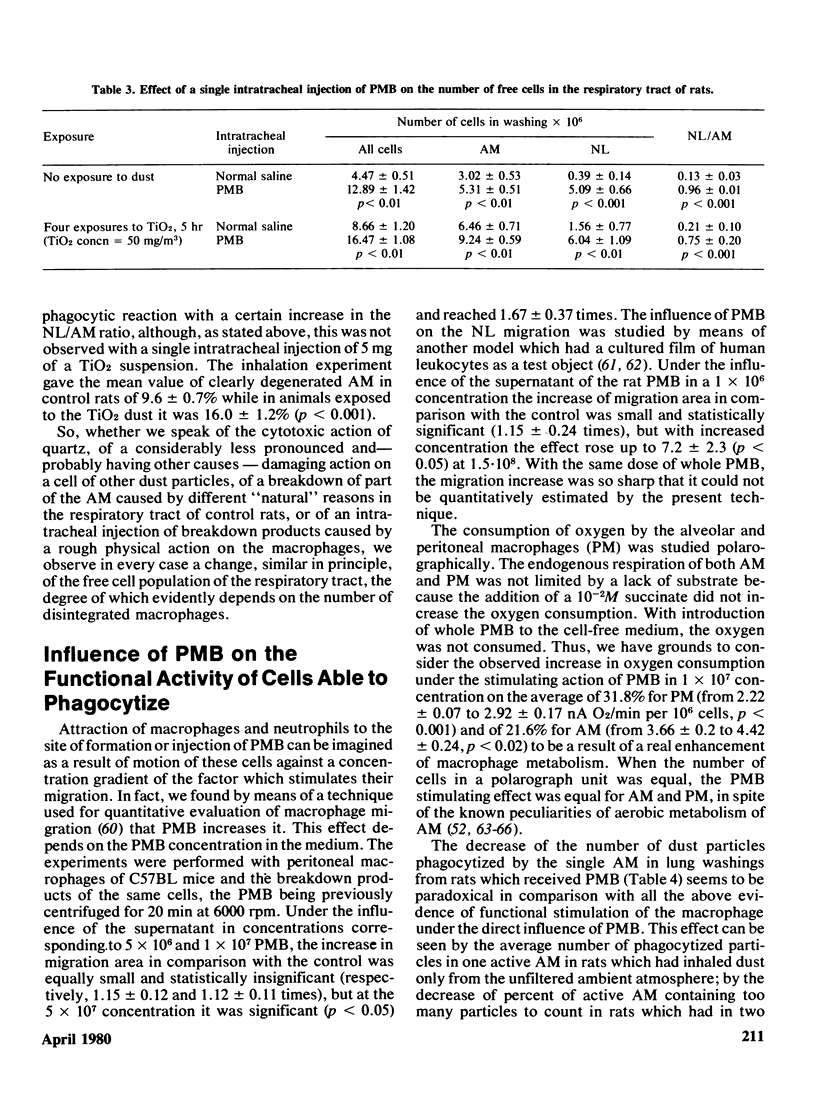
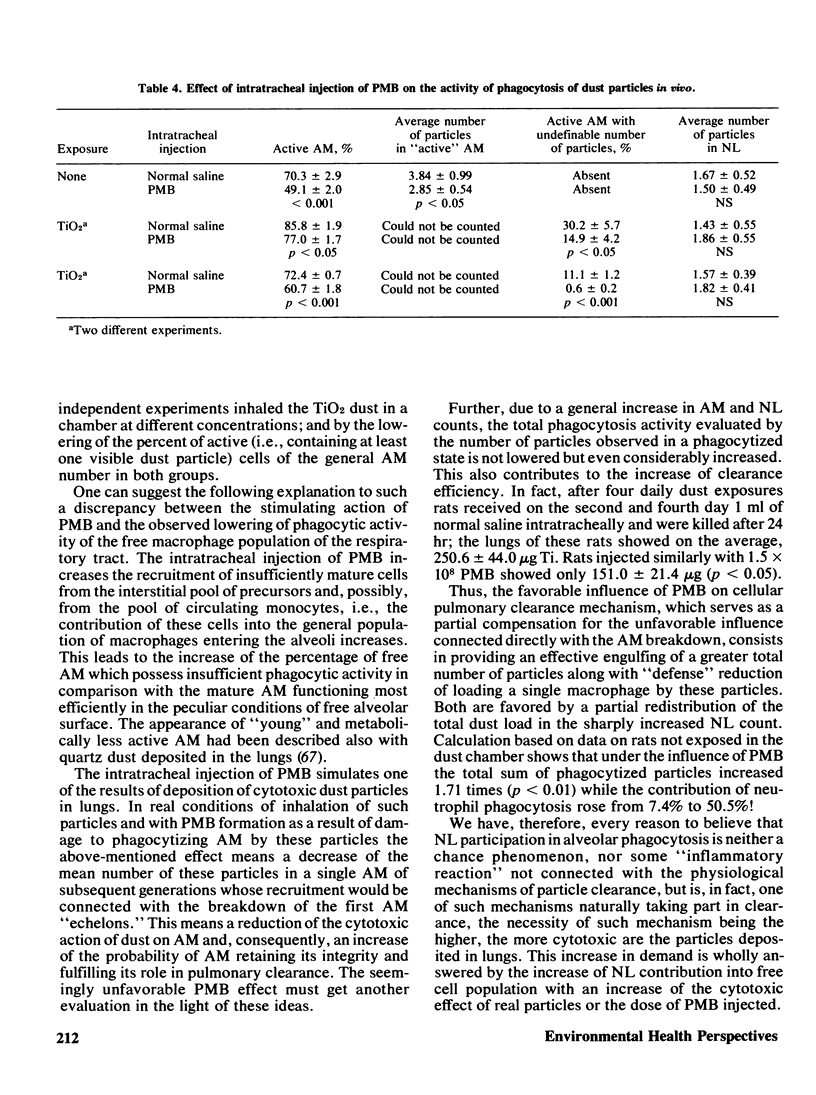
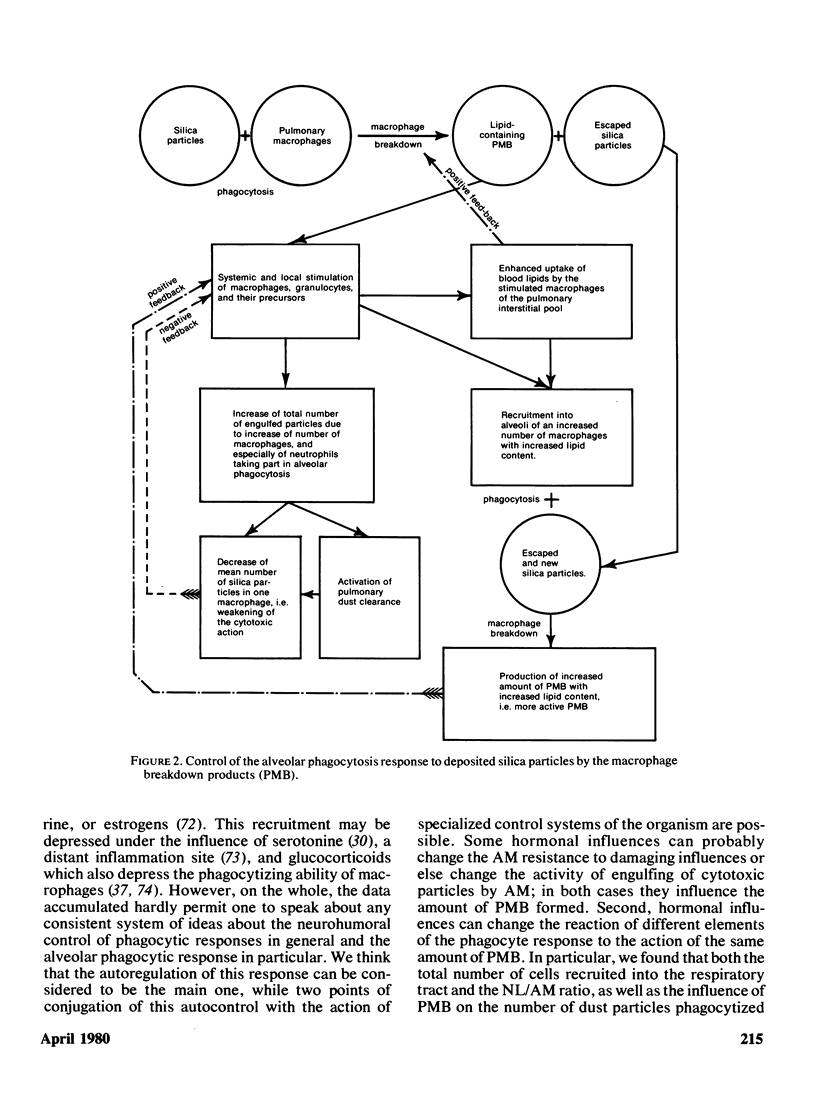
The adaptation of the alveolar phagocytosis response to the quantitative and qualitative features of dust deposited during inhalation consists not only in enhanced recruitment of alveolar macrophages (AM), but also in adding a more or less pronounced neutrophil leukocyte (NL) recruitment as an auxiliary participant of particle clearance. The NL contribution to clearance is especially typical for response to cytotoxic particles (quartz, in particular). An important feature of the adaptation considered is the limitation of the number of AM and NL recruited when an efficient clearance can be achieved by a lesser number of cells due to increased AM reistance to the damaging actin of phagocytized particles. The main mechanism providing the adequacy of the alveolar phagocytosis response is its self-regulation thrugh the products of macrophage breakdown (PMB). In a series of experiments with intraperitoneal and intratracheal injections of syngenetic PMB into rats and mice, it was shown that these products stimulate respiration and migration of phagocytic cells, their dose-dependent attraction to the site of PMB formation with the predominant NL contribution, increasing with the increase of amount of PMB, the AM and NL precursor cells recruitment from reserve pools, and the replenishment of these reserves in the process of hemopoiesis. At least some of the above effects are connected with the action of the lipid components of PMB. The action of specialized regulative systems of the organism can modify the response to PMB, judging by the results obtained by hydrocortisone injection. Autocontrol of alveolar phagocytosis requires great care in attempts at artificial stimulation of this process, as an excessive cell recruitment may promote the retention of particles in lungs.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E., Lippmann M. Factors influencing dust retention in the pulmonary parenchyma. Ann N Y Acad Sci. 1972 Dec 29;200:37–45. doi: 10.1111/j.1749-6632.1972.tb40176.x. [DOI] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y., Grantham W. G., Wyatt J. P. Origin of the lung macrophage. Evidence derived from radiation injury. Arch Pathol. 1969 Nov;88(5):540–546. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. The alveolar macrophage delivery system. Kinetic studies in cultured explants of murine lung. Am J Pathol. 1976 Apr;83(1):123–134. [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. The pulmonary interstitial cell as immediate precursor of the alveolar macrophage. Am J Pathol. 1972 Sep;68(3):521–537. [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H. The pulmonary macrophage. Environ Health Perspect. 1976 Aug;16:55–60. doi: 10.1289/ehp.761655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain J. D. Free cells in the lungs. Some aspects of their role, quantitation, and regulation. Arch Intern Med. 1970 Sep;126(3):477–487. doi: 10.1001/archinte.126.3.477. [DOI] [PubMed] [Google Scholar]
- Brain J. D. The effects of increased particles on the number of alveolar macrophages. Inhaled Part. 1970;1:209–225. [PubMed] [Google Scholar]
- Brain J. D. The respiratory tract and the environment. Environ Health Perspect. 1977 Oct;20:113–126. doi: 10.1289/ehp.7720113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell R., Anderson M. The induction of fibrogenesis by silica-treated alveolar macrophages. Environ Res. 1973 Dec;6(4):389–394. doi: 10.1016/0013-9351(73)90054-6. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I., Territo M. C., Golde D. W. UCLA Conference. Monocytes and macrophages: functions and diseases. Ann Intern Med. 1978 Jan;88(1):78–88. doi: 10.7326/0003-4819-88-1-78. [DOI] [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- GROSS P. THE PROCESSES INVOLVED IN THE BIOLOGIC ASPECTS OF PULMONARY DEPOSITION, CLEARANCE, AND RETENTION OF INSOLUBLE AEROSOLS. Health Phys. 1964 Dec;10:995–1002. doi: 10.1097/00004032-196412000-00018. [DOI] [PubMed] [Google Scholar]
- Green G. M. Alveolobronchiolar transport mechanisms. Arch Intern Med. 1973 Jan;131(1):109–114. [PubMed] [Google Scholar]
- Gross P., DeTreville T. P., Tolker E. B., Kaschak M., Babyak M. A. The pulmonary macrophage response to irritants. An attempt at quantitation. Arch Environ Health. 1969 Feb;18(2):174–185. doi: 10.1080/00039896.1969.10665390. [DOI] [PubMed] [Google Scholar]
- Gross P., Detreville R. T. The lung as an embattled domain against inanimate pollutants. A precis of mechanisms. Am Rev Respir Dis. 1972 Nov;106(5):684–691. doi: 10.1164/arrd.1972.106.5.684. [DOI] [PubMed] [Google Scholar]
- Kavet R. I., Brain J. D., Levens D. J. Characteristics of pulmonary macrophages lavaged from hamsters exposed to iron oxide aerosols. Lab Invest. 1978 Mar;38(3):312–319. [PubMed] [Google Scholar]
- Kilburn K. H. Acute bronchitis due to cotton plant polyphenols. Ann N Y Acad Sci. 1974;221:335–339. doi: 10.1111/j.1749-6632.1974.tb28235.x. [DOI] [PubMed] [Google Scholar]
- Kilburn K. H. Clearance zones in the distal lung. Ann N Y Acad Sci. 1974;221:276–281. doi: 10.1111/j.1749-6632.1974.tb28226.x. [DOI] [PubMed] [Google Scholar]
- Kilburn K. H., McKenzie W. Leukocyte recruitment to airways by cigarette smoke and particle phase in contrast to cytotoxicity of vapor. Science. 1975 Aug 22;189(4203):634–637. doi: 10.1126/science.1162344. [DOI] [PubMed] [Google Scholar]
- Klosterkötter W., Einbrodt H. J. Quantitative tierexperimentelle Untersuchungen über den Abtransport von Staub aus den Lungen in die regionalen Lymphknoten. Arch Hyg Bakteriol. 1965 May;149(3):367–384. [PubMed] [Google Scholar]
- LABELLE C. W., BRIEGER H. The fate of inhaled particles in the early postexposure period. II. The role of pulmonary phagocytosis. Arch Environ Health. 1960 Nov;1:423–427. doi: 10.1080/00039896.1960.10662717. [DOI] [PubMed] [Google Scholar]
- LEVIS F. Studio sul meccanismo di penetrazione della polvere nell'interstizio polmonare. I. Impostazione generale. Med Lav. 1956 Aug-Sep;47(8-9):465–473. [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., FARISS B. Lysozyme content of alveolar and peritoneal macrophages from the rabbit. J Immunol. 1961 Feb;86:133–136. [PubMed] [Google Scholar]
- Miller K., Kagan E. The in vivo effects of quartz on alveolar macrophage membrane topography and on the characteristics of the intrapulmonary cell population. J Reticuloendothel Soc. 1977 May;21(5):307–316. [PubMed] [Google Scholar]
- NAGELSCHMIDT G., NELSON E. S., KING E. J., ATTYGALLE D., YOGANATHAN M. The recovery of quartz and other minerals from the lungs of rats; a study in experimental silicosis. AMA Arch Ind Health. 1957 Sep;16(3):188–202. [PubMed] [Google Scholar]
- Nourse L. D., Nourse P. N., Botes H., Schwartz H. M. The effects of macrophages isolated from the lungs of guinea pigs dusted with silica on collagen biosynthesis by guinea pig fibroblasts in cell culture. Environ Res. 1975 Apr;9(2):115–127. doi: 10.1016/0013-9351(75)90056-0. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott F., Schlipköter H. W., Brockhaus A. Tierexperimentelle Grundlagen zur medizinischen Silikoseprophylaxe mit Polyvinylpyridin-N-oxid (P204) Dtsch Med Wochenschr. 1968 Dec 20;93(51):2479–2482. doi: 10.1055/s-0028-1110965. [DOI] [PubMed] [Google Scholar]
- Rasche B., Ulmer W. T. Die zelluläre Retention und der zelluläre Transport inhalierter Staubpartikel in Alveolarmakrophagen. Med Thorac. 1967;24(4):227–236. [PubMed] [Google Scholar]
- Rasche B., Ulmer W. T., Leder L. D. Zur Wirkung von Aluminiumchlorid-Aerosol auf die Reaktionen der Alveolarmakrophagen nach Quarzbestaubung. Int Arch Arbeitsmed. 1965 Jun 21;21(3):193–206. [PubMed] [Google Scholar]
- Rylander R., Snella M. C. Acute inhalation toxicity of cotton plant dusts. Br J Ind Med. 1976 Aug;33(3):175–180. doi: 10.1136/oem.33.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMINOVITCH L., MCCULLOCH E. A., TILL J. E. THE DISTRIBUTION OF COLONY-FORMING CELLS AMONG SPLEEN COLONIES. J Cell Physiol. 1963 Dec;62:327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- Simon L. M., Robin E. D., Phillips J. R., Acevedo J., Axline S. G., Theodore J. Enzymatic basis for bioenergetic differences of alveolar versus peritoneal macrophages and enzyme regulation by molecular O2. J Clin Invest. 1977 Mar;59(3):443–448. doi: 10.1172/JCI108658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. I., Stuart A. E. Effects of simple lipids on macrophages in vitro. J Pathol. 1975 Jan;115(1):13–16. doi: 10.1002/path.1711150103. [DOI] [PubMed] [Google Scholar]
- Snella M. C., Rylander R. Réactions cellulaires dans les poumons après inhalation de poussière de coton et d'endotoxine. Schweiz Med Wochenschr. 1977 Feb 12;107(6):198–200. [PubMed] [Google Scholar]
- Sorokin S. P., Brain J. D. Pathways of clearance in mouse lungs exposed to iron oxide aerosols. Anat Rec. 1975 Mar;181(3):581–625. doi: 10.1002/ar.1091810304. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Thomas E. D., Ramberg R. E., Sale G. E., Sparkes R. S., Golde D. W. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976 Jun 4;192(4243):1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- Tucker A. D., Wyatt J. H., Undery D. Clearance of inhaled particles from alveoli by normal interstitial drainage pathways. J Appl Physiol. 1973 Nov;35(5):719–732. doi: 10.1152/jappl.1973.35.5.719. [DOI] [PubMed] [Google Scholar]
- Volkman A. Disparity in origin of mononuclear phagocyte populations. J Reticuloendothel Soc. 1976 Apr;19(4):249–268. [PubMed] [Google Scholar]
- Walker R. F., Eidson G., Hatcher J. D. Influence of cotton dust inhalation on free lung cells in rats and guinea pigs. Lab Invest. 1975 Jul;33(1):28–32. [PubMed] [Google Scholar]


