Abstract
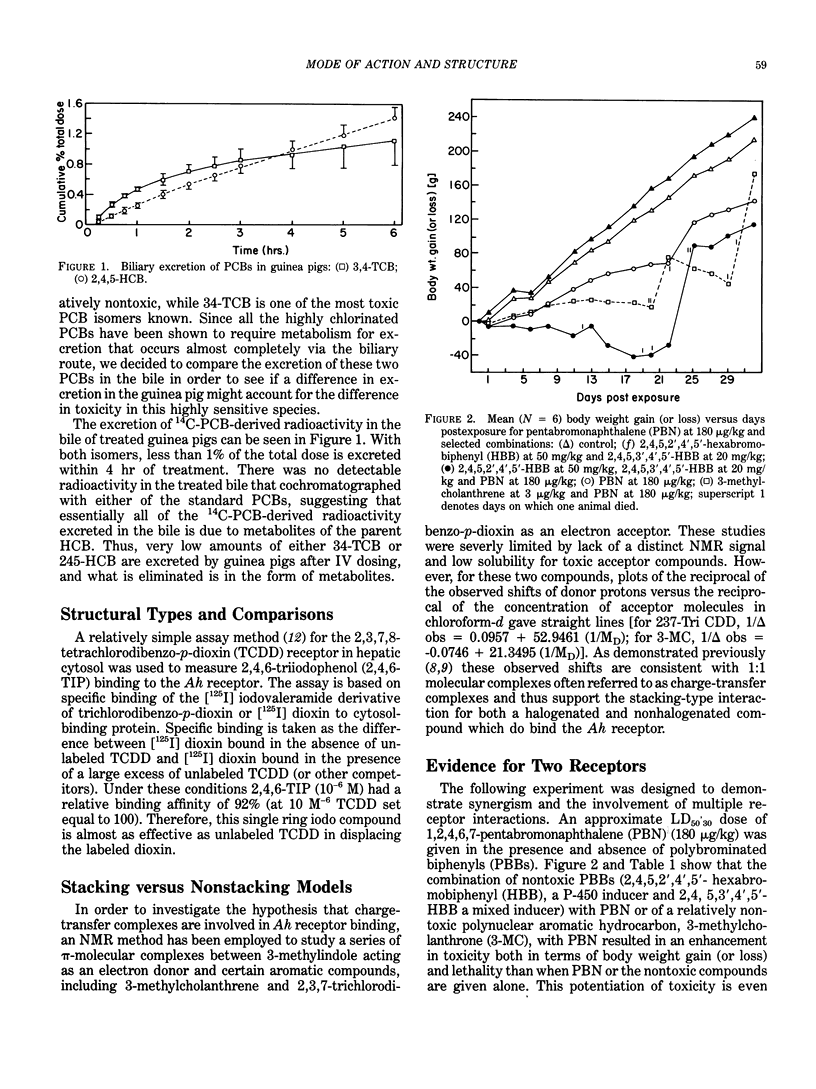
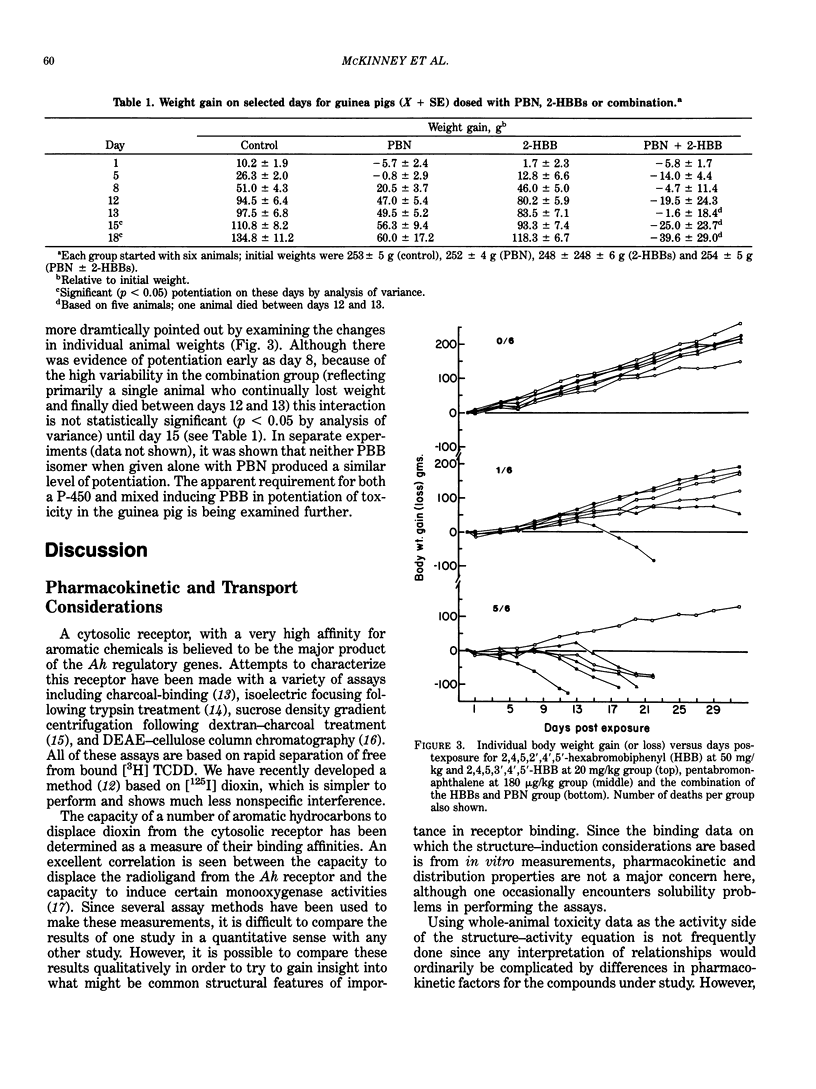
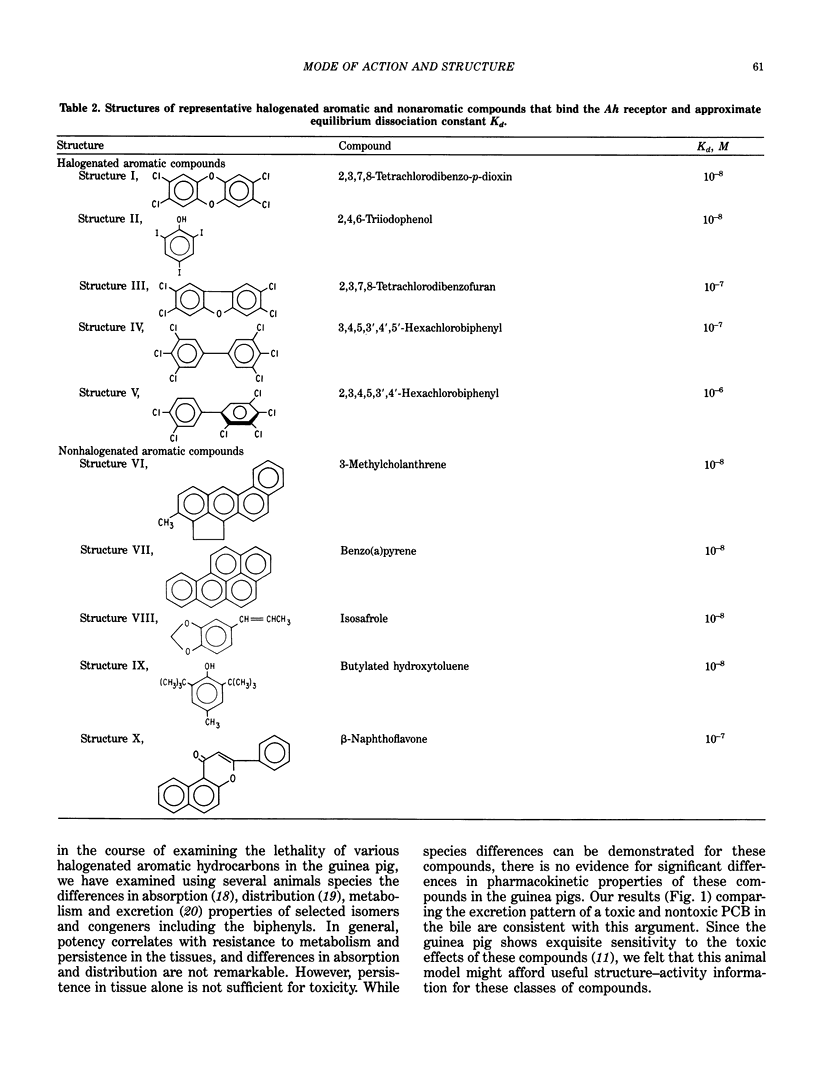
A comparison of the structure-induction (involving rat and mouse Ah receptor binding) and structure-toxicity (in vivo guinea pig toxicity) relationships suggests that two receptors with structurally distinct binding properties may be involved. This is supported by demonstration of potentiated toxicity through a mechanism believed to involve the Ah receptor as a site of loss with respect to toxicity. Theoretical and working models are proposed for these separate receptors to aid in the search for other relevant binding proteins. The findings suggest that polychlorinated biphenyls that are relatively low in toxicity may have modulating properties on the action of highly toxic compounds with which they are normally found in the environment.
Full text
PDF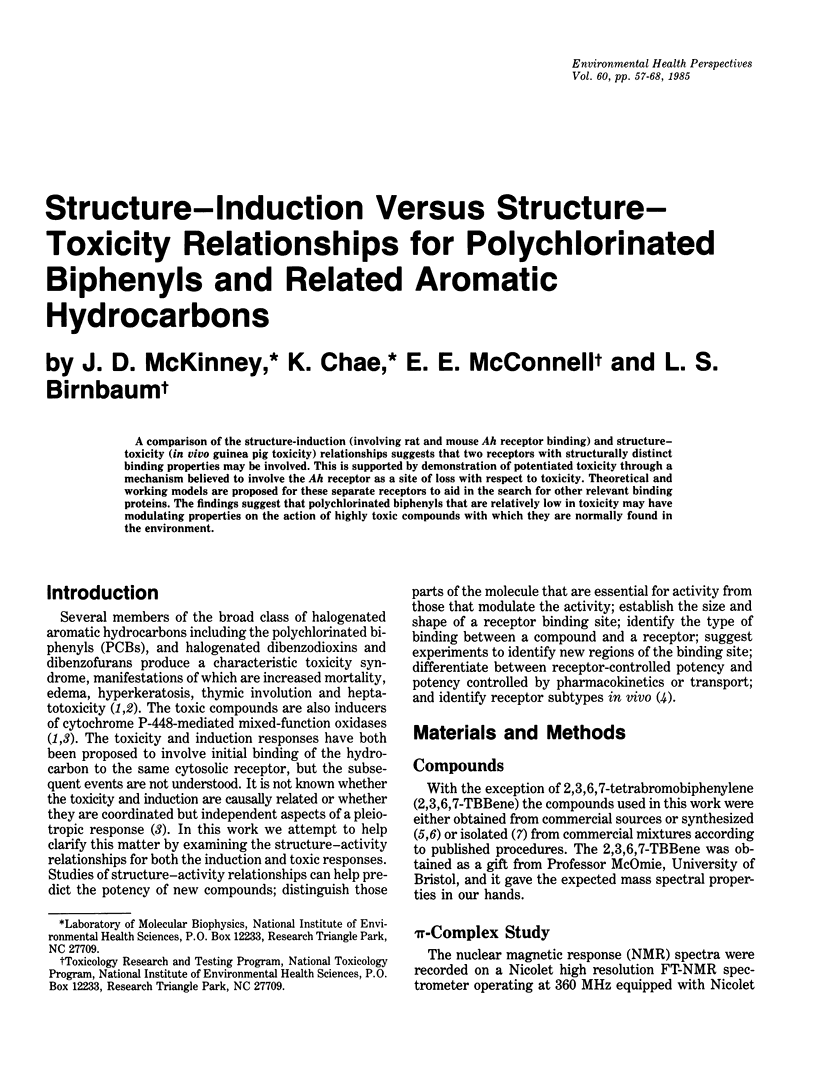




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albro P. W., Fishbein L. Intestinal absorption of polychlorinated biphenyls in rats. Bull Environ Contam Toxicol. 1972 Jul;8(1):26–31. doi: 10.1007/BF01684500. [DOI] [PubMed] [Google Scholar]
- Bandiera S., Safe S., Okey A. B. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982 Apr;39(3):259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Bigelow S. W., Nebert D. W. The Ah regulatory gene product. Survey of nineteen polycyclic aromatic compounds' and fifteen benzo[a]pyrene metabolites' capacity to bind to the cytosolic receptor. Toxicol Lett. 1982 Jan;10(1):109–118. doi: 10.1016/0378-4274(82)90276-4. [DOI] [PubMed] [Google Scholar]
- Birnbaum L. S., Darcey D. J., McKinney J. D. Hexabromonaphthalene contaminants of polybrominated biphenyls: chemical composition and disposition in the rat. J Toxicol Environ Health. 1983 Oct-Dec;12(4-6):555–573. doi: 10.1080/15287398309530449. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J. M., Elfström G., Högberg B., Gustafsson J. A. Ontogeny of the rat hepatic receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin and its endocrine indepence. Cancer Res. 1979 Nov;39(11):4653–4656. [PubMed] [Google Scholar]
- Chae K., Albro P. W., Luster M. I., McKinney J. D. A screening assay for the tetrachlorodibenzo-p-dioxin receptor using the [125I]iodovaleramide derivative of trichlorodibenzo-p-dioxin as the binding ligand. Int J Environ Anal Chem. 1984;17(3-4):267–274. doi: 10.1080/03067318408076978. [DOI] [PubMed] [Google Scholar]
- Courtney K. D., Moore J. A. Teratology studies with 2,4,5-trichlorophenoxyacetic acid and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1971 Nov;20(3):396–403. doi: 10.1016/0041-008x(71)90282-1. [DOI] [PubMed] [Google Scholar]
- Dannan G. A., Mileski G. J., Aust S. D. Purification of polybrominated biphenyl congeners. J Toxicol Environ Health. 1982 Mar;9(3):423–438. doi: 10.1080/15287398209530175. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A., McKinney J. D., Lucier G. W., Hickman P., Bergman H., Moore J. A. Toxicological assessment of hexachlorobiphenyl isomers and 2,3,7,8,-tetrachlorodibenzofuran in chicks. II. Effects on drug metabolism and porphyrin accumulation. Toxicol Appl Pharmacol. 1976 Apr;36(1):81–92. doi: 10.1016/0041-008x(76)90028-4. [DOI] [PubMed] [Google Scholar]
- Greenlee W. F., Poland A. Nuclear uptake of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J and DBA/2J mice. Role of the hepatic cytosol receptor protein. J Biol Chem. 1979 Oct 10;254(19):9814–9821. [PubMed] [Google Scholar]
- Hannah R. R., Nebert D. W., Eisen H. J. Regulatory gene product of the Ah complex. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3-methylcholanthrene binding to several moieties in mouse liver cytosol. J Biol Chem. 1981 May 10;256(9):4584–4590. [PubMed] [Google Scholar]
- Hook G. E., Haseman J. K., Lucier G. W. Induction and suppression of hepatic and extrahepatic microsomal foreign-compound-metabolizing enzyme systems by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem Biol Interact. 1975 Mar;10(3):199–214. doi: 10.1016/0009-2797(75)90113-1. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D. The toxicity of polychlorinated polycyclic compounds and related chemicals. CRC Crit Rev Toxicol. 1974 Jan;2(4):445–498. doi: 10.3109/10408447309025705. [DOI] [PubMed] [Google Scholar]
- McConnell E. E., Moore J. A., Haseman J. K., Harris M. W. The comparative toxicity of chlorinated dibenzo-p-dioxins in mice and guinea pigs. Toxicol Appl Pharmacol. 1978 May;44(2):335–356. doi: 10.1016/0041-008x(78)90195-3. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Singh P. Structure-activity relationships in halogenated biphenyls: unifying hypothesis for structural specificity. Chem Biol Interact. 1981 Jan;33(2-3):271–283. doi: 10.1016/0009-2797(81)90046-6. [DOI] [PubMed] [Google Scholar]
- Okey A. B., Bondy G. P., Mason M. E., Kahl G. F., Eisen H. J., Guenthner T. M., Nebert D. W. Regulatory gene product of the Ah locus. Characterization of the cytosolic inducer-receptor complex and evidence for its nuclear translocation. J Biol Chem. 1979 Nov 25;254(22):11636–11648. [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Pullman B., Pullman A. ELECTRON-DONOR AND -ACCEPTOR PROPERTIES OF BIOLOGICALLY IMPORTANT PURINES, PYRIMIDINES, PTERIDINES, FLAVINS, AND AROMATIC AMINO ACIDS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1197–1202. doi: 10.1073/pnas.44.12.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind A. B., Muschick H. Benoxaprofen suppression of polychlorinated biphenyl toxicity without alteration of mixed function oxidase function. Nature. 1983 Jun 9;303(5917):524–526. doi: 10.1038/303524a0. [DOI] [PubMed] [Google Scholar]
- Ross R. T., Biros F. J. Molecular complexes of 1,1,1-trichloro-2,2-bis (p-chlorophenyl) ethane with aromatic donors. Biochem Biophys Res Commun. 1970 May 22;39(4):723–731. doi: 10.1016/0006-291x(70)90265-2. [DOI] [PubMed] [Google Scholar]
- VELDSTRA H. Synergism and potentiation with special reference to the combination of structural analogues. Pharmacol Rev. 1956 Sep;8(3):339–387. [PubMed] [Google Scholar]


