Abstract
Nitroaromatic compounds, ArNO2 have widespread actual or potential use in medicine and cancer therapy. There is direct proof that free-radical metabolites are involved in many applications, and an appreciation of the conceptual basis for their therapeutic differential; however, an understanding of the detailed mechanisms involved is lacking. Redox properties control most biological responses of nitro compounds, and the characteristics of the one-electron couple: ArNO2/ArNO2- are detailed. The "futile metabolism" of nitroaryl compounds characteristic of most aerobic nitroreductase systems reflects competition between natural radical-decay pathways and a one-electron transfer reaction to yield superoxide ion, O2-. Prototropic properties control the rate of radical decay, and redox properties control the rate of electron transfer to O2 or other acceptors. There are clear parallels in the chemistry of ArNO2- and O2-. While nitro radicals have frequently been invoked as damaging species, they are very unreactive (except as simple reductants). It seems likely that reductive metabolism of nitroaryl compounds, although generally involving nitro radical-anions as obligate intermediates (and this is required for therapeutic selectivity towards anaerobes), results in biological damage via reductive metabolites of higher reduction order than the one-electron product.
Full text
PDF

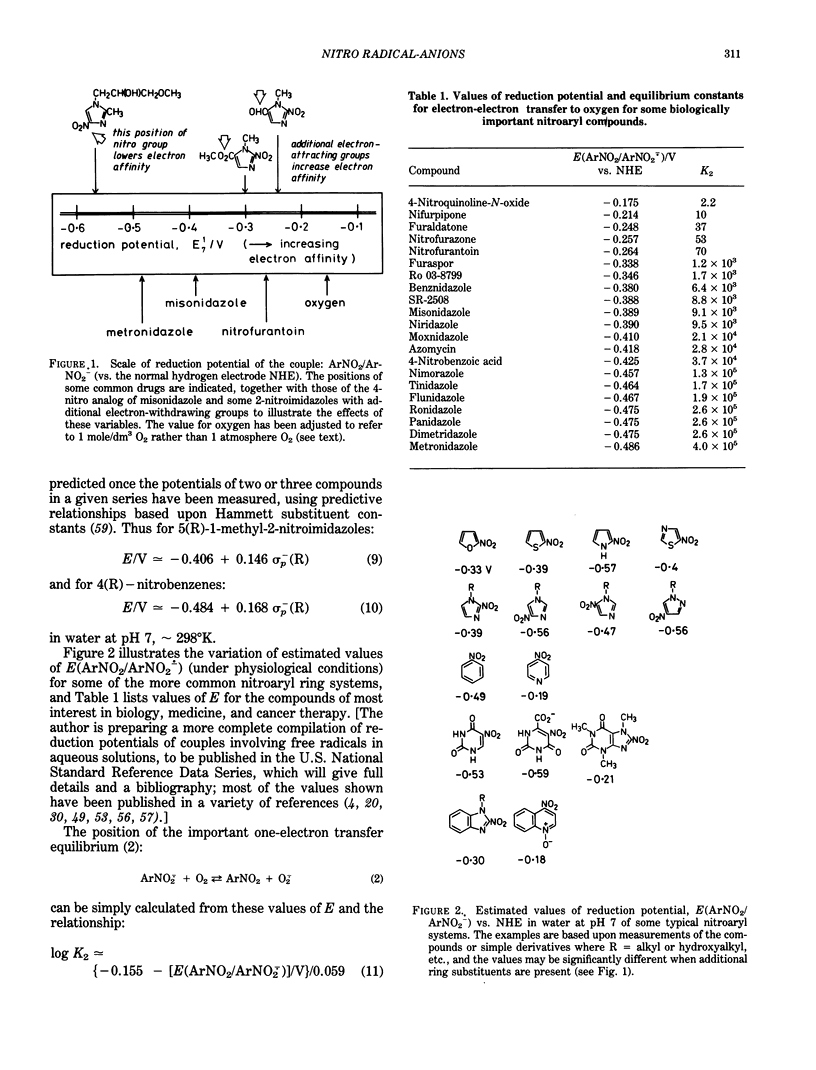


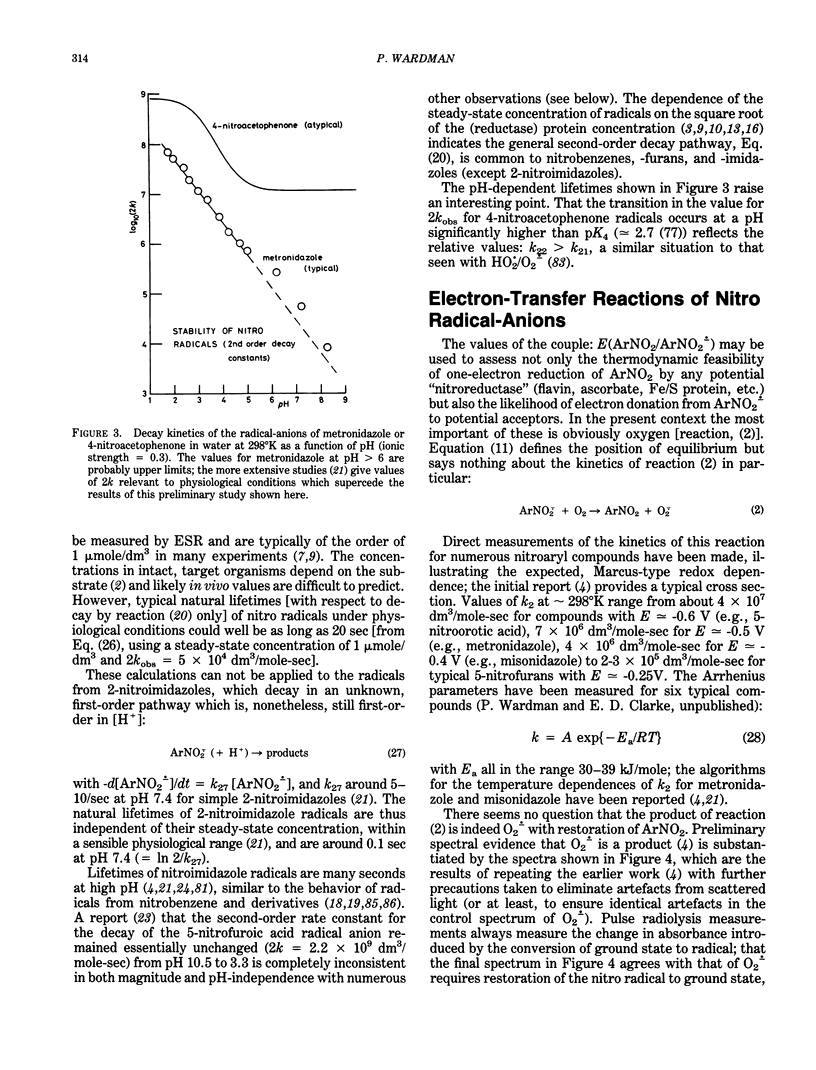
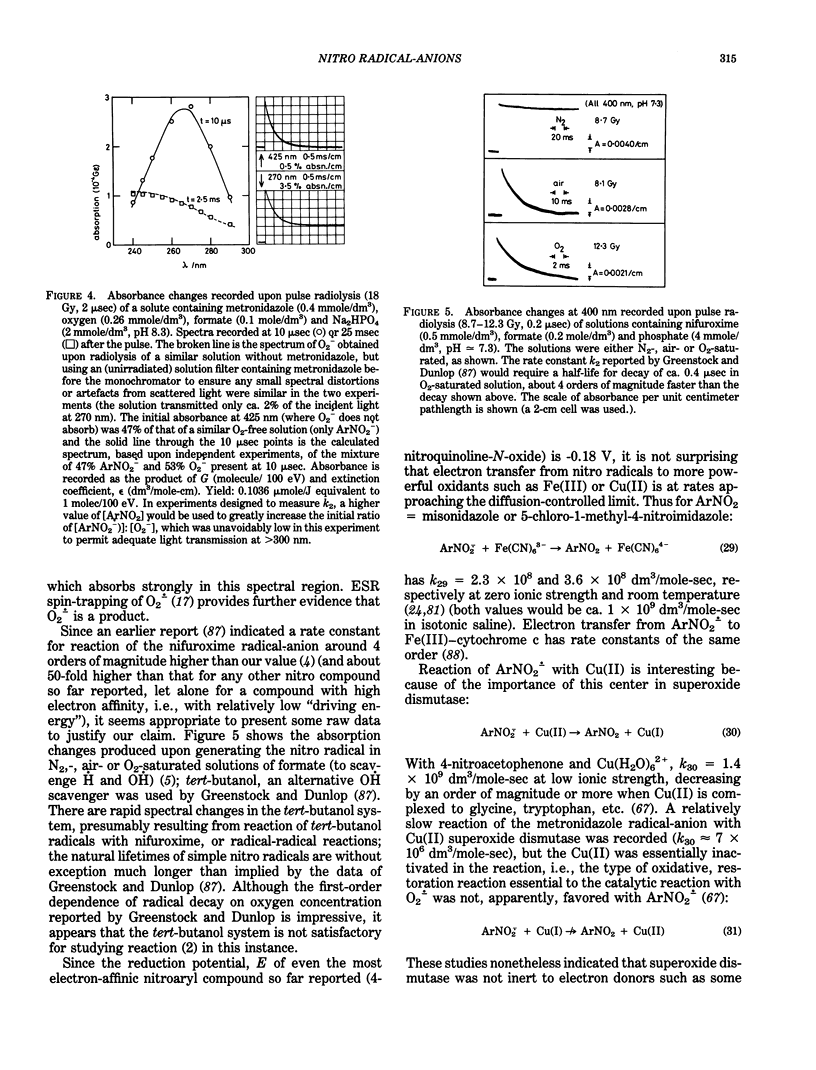





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. F. Energetics of the one-electron reduction steps of riboflavin, FMN and FAD to their fully reduced forms. Biochim Biophys Acta. 1983 Jan 13;722(1):158–162. doi: 10.1016/0005-2728(83)90169-x. [DOI] [PubMed] [Google Scholar]
- Biaglow J. E. Cellular electron transfer and radical mechanisms for drug metabolism. Radiat Res. 1981 May;86(2):212–242. [PubMed] [Google Scholar]
- Biaglow J. E., Durand R. E. The effects of nitrobenzene derivatives on oxygen utilization and radiation response of an in vitro tumor model. Radiat Res. 1976 Mar;65(3):529–539. [PubMed] [Google Scholar]
- Biaglow J. E., Jacobson B., Koch C. The catalytic effect of the carcinogen "4-nitroquinoline-N-oxide" on the oxidation of vitamin C. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1316–1323. doi: 10.1016/0006-291x(76)91046-9. [DOI] [PubMed] [Google Scholar]
- Biaglow J. E., Jacobson B., Varnes M. The oxidation of ascorbate by electron affinic drugs and carcinogens. Photochem Photobiol. 1978 Oct-Nov;28(4-5):869–876. doi: 10.1111/j.1751-1097.1978.tb07034.x. [DOI] [PubMed] [Google Scholar]
- Breccia A., Berrilli G., Roffia S. Chemical radiosensitization of hypoxic cells and redox potentials: correlation of voltammetric results with pulse radiolysis data of nitro-compounds and radiosensitizers. Int J Radiat Biol Relat Stud Phys Chem Med. 1979 Jul;36(1):85–89. doi: 10.1080/09553007914550841. [DOI] [PubMed] [Google Scholar]
- Chemical modifiers of cancer treatment. Part 1. Banff, Canada, 27 November-1 December, 1983. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1161–1482. [PubMed] [Google Scholar]
- Clarke E. D., Wardman P. Are ortho-substituted 4-nitroimidazoles a new generation of radiation-induced arylating agents? Int J Radiat Biol Relat Stud Phys Chem Med. 1980 Apr;37(4):463–468. doi: 10.1080/09553008014550561. [DOI] [PubMed] [Google Scholar]
- Clarke E. D., Wardman P., Goulding K. H. Anaerobic reduction of nitroimidazoles by reduced flavin mononucleotide and by xanthine oxidase. Biochem Pharmacol. 1980 Oct 1;29(19):2684–2687. doi: 10.1016/0006-2952(80)90087-8. [DOI] [PubMed] [Google Scholar]
- Clarke E. D., Wardman P., Wilson I. Cis-trans isomerization of the (5-nitro-2-furyl)acrylamide, AF-2, initiated by ascorbate, glutathione, Fe(II) and OH-. Biochem Pharmacol. 1984 Jan 1;33(1):83–87. doi: 10.1016/0006-2952(84)90373-3. [DOI] [PubMed] [Google Scholar]
- Conference on chemical modification: radiation and cytotoxic drugs. Key Biscayne, Florida, 17-20 September, 1981. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):323–815. [PubMed] [Google Scholar]
- Docampo R., Mason R. P., Mottley C., Muniz R. P. Generation of free radicals induced by nifurtimox in mammalian tissues. J Biol Chem. 1981 Nov 10;256(21):10930–10933. [PubMed] [Google Scholar]
- Docampo R., Moreno S. N., Stoppani A. O., Leon W., Cruz F. S., Villalta F., Muniz R. F. Mechanism of nifurtimox toxicity in different forms of Trypanosoma cruzi. Biochem Pharmacol. 1981 Jul 15;30(14):1947–1951. doi: 10.1016/0006-2952(81)90204-5. [DOI] [PubMed] [Google Scholar]
- Duran R. E., Biaglow J. E., Sutherland R. M. Letter: Hypoxic radiosensitizers and cellular respiration. Br J Radiol. 1976 Jun;49(582):567–568. doi: 10.1259/0007-1285-49-582-567. [DOI] [PubMed] [Google Scholar]
- Edwards D. I. Mechanisms of cytotoxicity of nitroimidazole drugs. Prog Med Chem. 1981;18:87–116. doi: 10.1016/s0079-6468(08)70317-5. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Watts M. E., Patel K. B., Adams G. E. Sensitization of ultraviolet radiation damage in bacteria and mammalian cells. Br J Cancer Suppl. 1978 Jun;3:111–114. [PMC free article] [PubMed] [Google Scholar]
- Greenstock C. L., Dunlop I. Pulse radiolysis studies of nitrofurans: chemical radiosensitization. Radiat Res. 1973 Dec;56(3):428–440. [PubMed] [Google Scholar]
- Greenstock C. L., Ruddock G. W., Neta P. Rulse radiolysis and ESR studies of the electron-affinic properties of nitroheterocyclic radiosensitizers. Radiat Res. 1976 Jun;66(3):472–484. [PubMed] [Google Scholar]
- Hall E. J., Roizin-Towle L. Hypoxic sensitizers: radiobiological studies at the cellular level. Radiology. 1975 Nov;117(2):453–457. doi: 10.1148/117.2.453. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B., Mason R. P., Rowlett R., Kispert L. D. An electron spin resonance investigation and molecular orbital calculation of the anion radical intermediate in the enzymatic cis-trans isomerization of furylfuramide, a nitrofuran derivative of ethylene. Biochim Biophys Acta. 1981 Jul 24;660(1):102–109. doi: 10.1016/0005-2744(81)90114-5. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B., Perez-Reyes E., Mason R. P., Peterson F. J., Holtzman J. L. Electron spin resonance evidence for a free radical intermediate in the cis-trans isomerization of furylfuramide by oxygen-sensitive nitroreductases. Mol Pharmacol. 1979 Nov;16(3):1059–1064. [PubMed] [Google Scholar]
- Ma H., Hardy C. R., O'Neill P. Formation of halide-ions on one-electron reduction of halogenated nitroimidazoles in aqueous solution. A radiolytic study. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Feb;41(2):151–160. doi: 10.1080/09553008214550161. [DOI] [PubMed] [Google Scholar]
- Masana M., de Toranzo E. G., Castro J. A. Reductive metabolism and activation of benznidazole. Biochem Pharmacol. 1984 Apr 1;33(7):1041–1045. doi: 10.1016/0006-2952(84)90511-2. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Holtzman J. L. The mechanism of microsomal and mitochondrial nitroreductase. Electron spin resonance evidence for nitroaromatic free radical intermediates. Biochemistry. 1975 Apr 22;14(8):1626–1632. doi: 10.1021/bi00679a013. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Holtzman J. L. The role of catalytic superoxide formation in the O2 inhibition of nitroreductase. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1267–1274. doi: 10.1016/0006-291x(75)90163-1. [DOI] [PubMed] [Google Scholar]
- McClelland R. A., Fuller J. R., Seaman N. E., Rauth A. M., Battistella R. 2-Hydroxylaminoimidazoles--unstable intermediates in the reduction of 2-nitroimidazoles. Biochem Pharmacol. 1984 Jan 15;33(2):303–309. doi: 10.1016/0006-2952(84)90489-1. [DOI] [PubMed] [Google Scholar]
- Mohindra J. K., Rauth A. M. Increased cell killing by metronidazole and nitrofurazone of hypoxic compared to aerobic mammalian cells. Cancer Res. 1976 Mar;36(3):930–936. [PubMed] [Google Scholar]
- Moreno S. N., Docampo R., Mason R. P., Leon W., Stoppani A. O. Different behaviors of benznidazole as free radical generator with mammalian and Trypanosoma cruzi microsomal preparations. Arch Biochem Biophys. 1982 Oct 15;218(2):585–591. doi: 10.1016/0003-9861(82)90383-6. [DOI] [PubMed] [Google Scholar]
- Moreno S. N., Docampo R. Mechanism of toxicity of nitro compounds used in the chemotherapy of trichomoniasis. Environ Health Perspect. 1985 Dec;64:199–208. doi: 10.1289/ehp.8564199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S. N., Mason R. P., Docampo R. Distinct reduction of nitrofurans and metronidazole to free radical metabolites by Tritrichomonas foetus hydrogenosomal and cytosolic enzymes. J Biol Chem. 1984 Jul 10;259(13):8252–8259. [PubMed] [Google Scholar]
- Moreno S. N., Mason R. P., Muniz R. P., Cruz F. S., Docampo R. Generation of free radicals from metronidazole and other nitroimidazoles by Tritrichomonas foetus. J Biol Chem. 1983 Apr 10;258(7):4051–4054. [PubMed] [Google Scholar]
- Olive P. L. Correlation between metabolic reduction rates and electron affinity of nitroheterocycles. Cancer Res. 1979 Nov;39(11):4512–4515. [PubMed] [Google Scholar]
- Perez-Reyes E., Kalyanaraman B., Mason R. P. The reductive metabolism of metronidazole and ronidazole by aerobic liver microsomes. Mol Pharmacol. 1980 Mar;17(2):239–244. [PubMed] [Google Scholar]
- Peterson F. J., Mason R. P., Hovsepian J., Holtzman J. L. Oxygen-sensitive and -insensitive nitroreduction by Escherichia coli and rat hepatic microsomes. J Biol Chem. 1979 May 25;254(10):4009–4014. [PubMed] [Google Scholar]
- Polnaszek C. F., Peterson F. J., Holtzman J. L., Mason R. P. No detectable reaction of the anion radical metabolite of nitrofurans with reduced glutathione or macro-molecules. Chem Biol Interact. 1984 Oct;51(3):263–271. doi: 10.1016/0009-2797(84)90152-2. [DOI] [PubMed] [Google Scholar]
- Raleigh J. A., Liu S. F. Reductive fragmentation of 2-nitroimidazoles in the presence of nitroreductases--glyoxal formation from misonidazole. Biochem Pharmacol. 1983 Apr 15;32(8):1444–1446. doi: 10.1016/0006-2952(83)90460-4. [DOI] [PubMed] [Google Scholar]
- Raleigh J. A., Shum F. Y., Liu S. F. Nitroreductase-induced binding of nitroaromatic radiosensitizers to unsaturated lipids. Nitroxyl adducts. Biochem Pharmacol. 1981 Nov 1;30(21):2921–2925. doi: 10.1016/0006-2952(81)90253-7. [DOI] [PubMed] [Google Scholar]
- Rauth A. M., McClelland R. A., Michaels H. B., Battistella R. The oxygen dependence of the reduction of nitroimidazoles in a radiolytic model system. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1323–1326. doi: 10.1016/0360-3016(84)90341-9. [DOI] [PubMed] [Google Scholar]
- Reynolds A. V. The activity of nitro-compounds against Bacteroides fragilis is related to their electron affinity. J Antimicrob Chemother. 1981 Aug;8(2):91–99. doi: 10.1093/jac/8.2.91. [DOI] [PubMed] [Google Scholar]
- Sealy R. C., Swartz H. M., Olive P. L. Electron spin resonance-spin trapping. Detection of superoxide formation during aerobic microsomal reduction of nitro-compounds. Biochem Biophys Res Commun. 1978 May 30;82(2):680–684. doi: 10.1016/0006-291x(78)90928-2. [DOI] [PubMed] [Google Scholar]
- Smith B. R. Hypoxia-enhanced reduction and covalent binding of [2-3H]misonidazole in the perfused rat liver. Biochem Pharmacol. 1984 Apr 15;33(8):1379–1381. doi: 10.1016/0006-2952(84)90199-0. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Selective chemotherapy of noncycling cells in an in vitro tumor model. Cancer Res. 1974 Dec;34(12):3501–3503. [PubMed] [Google Scholar]
- Tatsumi K., Kitamur S., Kog N., Yoshimura H., Kato Y. Cis-trans isomerization of nitrofuran derivatives by xanthine oxidase. Biochem Biophys Res Commun. 1976 Dec 20;73(4):947–952. doi: 10.1016/0006-291x(76)90213-8. [DOI] [PubMed] [Google Scholar]
- Tatsumi K., Koga N., Kitamura S., Yoshimura H., Wardman P., Kato Y. Enzymic cis-trans isomerization of nitrofuran derivatives: isomerizing activity of xanthine oxidase, lipoyl dehydrogenase, DT-diaphorase and liver microsomes. Biochim Biophys Acta. 1979 Mar 16;567(1):75–87. doi: 10.1016/0005-2744(79)90174-8. [DOI] [PubMed] [Google Scholar]
- Varghese A. J. Glutathione conjugates of misonidazole. Biochem Biophys Res Commun. 1983 May 16;112(3):1013–1020. doi: 10.1016/0006-291x(83)91719-9. [DOI] [PubMed] [Google Scholar]
- Varghese A. J., Whitmore G. F. Detection of a reactive metabolite of misonidazole in hypoxic mammalian cells. Radiat Res. 1984 Feb;97(2):262–271. [PubMed] [Google Scholar]
- Wardman P., Clarke E. D. Oxygen inhibition of nitroreductase: electron transfer from nitro radical-anions to oxygen. Biochem Biophys Res Commun. 1976 Apr 19;69(4):942–949. doi: 10.1016/0006-291x(76)90464-2. [DOI] [PubMed] [Google Scholar]
- Wardman P. Letter: Protonation of the radical-anions of nitro-imidazole radiosensitizers and the formation of radical-adducts. Int J Radiat Biol Relat Stud Phys Chem Med. 1975 Dec;28(6):585–588. doi: 10.1080/09553007514551441. [DOI] [PubMed] [Google Scholar]
- Wardman P. The use of nitroaromatic compounds as hypoxic cell radiosensitizers. Curr Top Radiat Res Q. 1977 Aug;11(4):347–398. [PubMed] [Google Scholar]
- Weis W. Ascorbic acid and biological systems. Ascorbic acid and electron transport. Ann N Y Acad Sci. 1975 Sep 30;258:190–200. doi: 10.1111/j.1749-6632.1975.tb29279.x. [DOI] [PubMed] [Google Scholar]
- Willson R. L., Searle A. J. Metronidazole (Flagyl): iron catalysed reaction with sulphydryl groups and tumour radiosensitisation. Nature. 1975 Jun 5;255(5508):498–500. doi: 10.1038/255498a0. [DOI] [PubMed] [Google Scholar]
- Yarlett N., Gorrell T. E., Marczak R., Müller M. Reduction of nitroimidazole derivatives by hydrogenosomal extracts of Trichomonas vaginalis. Mol Biochem Parasitol. 1985 Jan;14(1):29–40. doi: 10.1016/0166-6851(85)90103-3. [DOI] [PubMed] [Google Scholar]


