Abstract
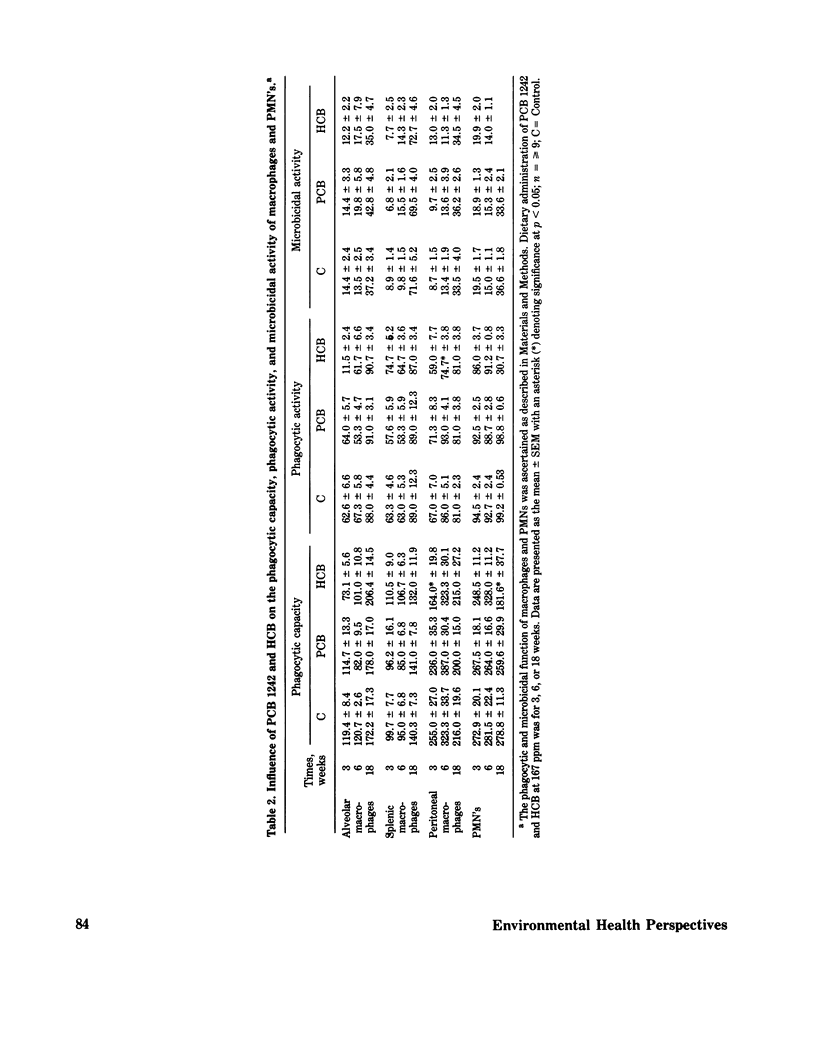
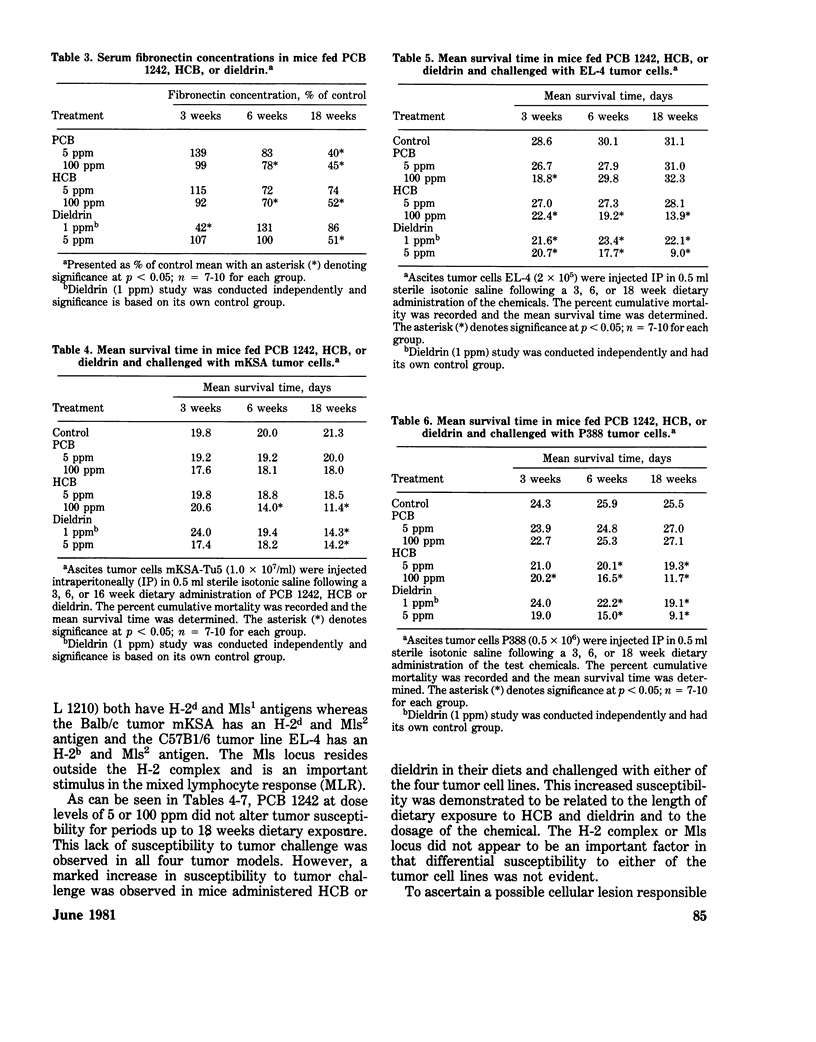
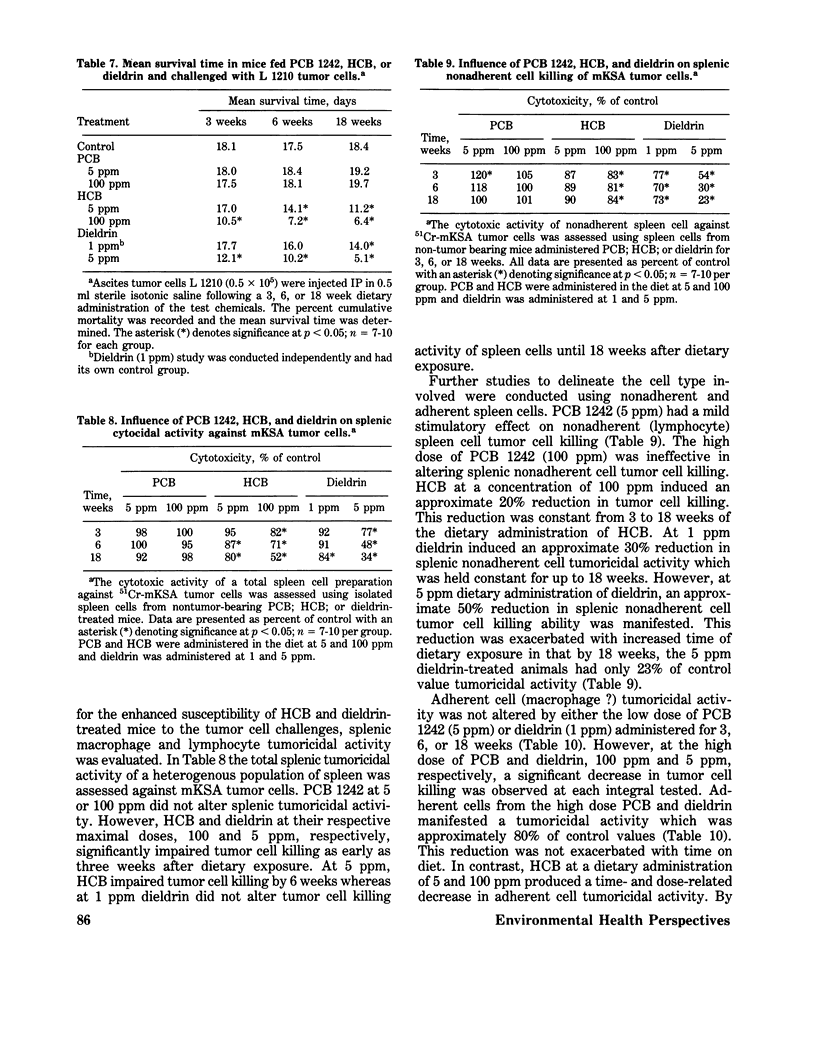
Immunomodulation by environmental chemical contaminants and the role immune parameters play in toxicity and risk assessment studies is of increasing concern. Although considerable evidence has indicated that various xenobiotics may be immunosuppressive, little attention has been directed toward ascertaining a specific cellular locus which could be responsible for the impaired immune responsiveness. Since previous studies had suggested a macrophage defect in xenobiotic-induced immunosuppression and since macrophages are integral components of an immune response, an in-depth evaluation of macrophage function was conducted in xenobiotic-exposed mice. Macrophages isolated from mice receiving PCB, HCB, and dieldrin had no alteration in their in vitro O2 consumption while at rest or during phagocytosis. In addition, no alteration in in vitro phagocytic activity, phagocytic capacity or microbicidal activity was demonstrated. However, a significant impairment in the in vivo phagocytic clearance of a labelled antigen and an altered tissue distribution of the antigen was observed and was, perhaps, related, in part, to a significant decrease in serum fibronectin, an opsonic alpha 2 surface-binding glycoprotein. Furthermore, animals exposed to HCB and dieldrin, but not to PCB, had a profound decrease in their resistance to a challenge tumor cell implant which was related to a select alteration in tumor cell killing. The adherent spleen cells from HCB-treated mice had a profound suppression in their tumoricidal activity which was in contrast to dieldrin-treated mice, where the target cell type appeared to be the nonadherent cells. However, although dieldrin-exposed adherent cells (macrophages ?) did nt appear to have an altered tumoricidal capacity, all four macrophage types isolated from dieldrin-treated mice had a significantly impaired ability to process a cellular antigen. Splenic and alveolar macrophages appeared to be the most sensitive cell types to dieldrin. The present studies suggest that macrophage dysfunction may be an integral part of xenobiotic-induced immunosuppression and that the effector but not affector component of macrophage function may be the site of alteration.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autrup H., Harris C. C., Stoner G. D., Selkirk J. K., Schafer P. W., Trump B. F. Metabolism of [3H]benzo[a]pyrene by cultured human bronchus and cultured human pulmonary alveolar macrophages. Lab Invest. 1978 Mar;38(3):217–224. [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Brunda M. J., Raffel S. Macrophage processing of antigen for induction of tumor immunity. Cancer Res. 1977 Jun;37(6):1838–1844. [PubMed] [Google Scholar]
- Canty T. G., Wunderlich J. R. Quantitative in vitro assay of cytotoxic cellular immunity. J Natl Cancer Inst. 1970 Oct;45(4):761–772. [PubMed] [Google Scholar]
- Chapman H. A., Jr, Hibbs J. B., Jr Modulation of macrophage tumoricidal capability by components of normal serum: a central role for lipid. Science. 1977 Jul 15;197(4300):282–285. doi: 10.1126/science.195338. [DOI] [PubMed] [Google Scholar]
- Dean J. H., Padarathsingh M. L., Jerrells T. R., Keys L., Northing J. W. Assessment of immunobiological effects induced by chemicals, drugs or food additives. II. Studies with cyclophosphamide. Drug Chem Toxicol. 1979;2(1-2):133–153. doi: 10.3109/01480547908993186. [DOI] [PubMed] [Google Scholar]
- FISHMAN M., HAMMERSTROM R. A., BOND V. P. In vitro transfer of macrophage RNA to lymph node cells. Nature. 1963 May 11;198:549–551. doi: 10.1038/198549a0. [DOI] [PubMed] [Google Scholar]
- FREI P. C., BENACERRAF B., THORBECKE G. J. PHAGOCYTOSIS OF THE ANTIGEN, A CRUCIAL STEP IN THE INDUCTION OF THE PRIMARY RESPONSE. Proc Natl Acad Sci U S A. 1965 Jan;53:20–23. doi: 10.1073/pnas.53.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzl R. E., McMaster P. D. The primary immune response in mice. I. The enhancement and suppression of hemolysin production by a bacterial endotoxin. J Exp Med. 1968 Jun 1;127(6):1087–1107. doi: 10.1084/jem.127.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOBLE F. C., SINGER I. The reticuloendothelial system in experimental malaria and trypanosomiasis. Ann N Y Acad Sci. 1960 Jun 21;88:149–171. doi: 10.1111/j.1749-6632.1960.tb20016.x. [DOI] [PubMed] [Google Scholar]
- GRAY S. J., STERLING K. The tagging of red cells and plasma proteins with radioactive chromium. J Clin Invest. 1950 Dec;29(12):1604–1613. doi: 10.1172/JCI102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer J. H., Pry T. W. Effects of arsenicals on viral infections in mice. Am J Vet Res. 1972 Nov;33(11):2299–2307. [PubMed] [Google Scholar]
- Hamilton H. E., Morgan D. P., Simmons A. A pesticide (dieldrin)-induced immunohemolytic anemia. Environ Res. 1978 Oct;17(2):155–164. doi: 10.1016/0013-9351(78)90018-x. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Ivatt R. J. Functional and biochemical parameters of activation related to macrophage cytostatic effects on tumor cells. Int J Cancer. 1974 Nov 15;14(5):675–683. doi: 10.1002/ijc.2910140515. [DOI] [PubMed] [Google Scholar]
- Koller L. D., Exon J. H., Roan J. G. Humoral antibody response in mice after single dose exposure to lead or cadmium. Proc Soc Exp Biol Med. 1976 Feb;151(2):339–342. doi: 10.3181/00379727-151-39205. [DOI] [PubMed] [Google Scholar]
- Koller L. D. Immunosuppression produced by lead, cadmium, and mercury. Am J Vet Res. 1973 Nov;34(11):1457–1458. [PubMed] [Google Scholar]
- Koller L. D., Thigpen J. E. Biphenyl-exposed rabbits. Am J Vet Res. 1973 Dec;34(12):1605–1606. [PubMed] [Google Scholar]
- LITCHFIELD J. T., Jr A method for rapid graphic solution of time-per cent effect curves. J Pharmacol Exp Ther. 1949 Dec;97(4):399-408, 3 tab. [PubMed] [Google Scholar]
- Loose L. D., Silkworth J. B., Mudzinski S. P., Pittman K. A., Benitz K. F., Mueller W. Modification of the immune response by organochlorine xenobiotics. Drug Chem Toxicol. 1979;2(1-2):111–132. doi: 10.3109/01480547908993185. [DOI] [PubMed] [Google Scholar]
- Loose L. D., Silkworth J. B., Pittman K. A., Benitz K. F., Mueller W. Impaired host resistance to endotoxin and malaria in polychlorinated biphenyl- and hexachlorobenzene-treated mice. Infect Immun. 1978 Apr;20(1):30–35. doi: 10.1128/iai.20.1.30-35.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose L. D., Silkworth J. B., Warrington D. Cadmium-induced depression of the respiratory burst in mouse pulmonary alveolar macrophages, peritoneal macrophages and polymorphonuclear neutrophils. Biochem Biophys Res Commun. 1977 Nov 7;79(1):326–332. doi: 10.1016/0006-291x(77)90099-7. [DOI] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlan R. I., Burns W. H., White D. O. Two cytotoxic cells in peritoneal cavity of virus-infected mice: antibody-dependent macrophages and nonspecific killer cells. J Immunol. 1977 Nov;119(5):1569–1574. [PubMed] [Google Scholar]
- Mills G., Monticone V., Paetkau The role of macrophages in thymocyte mitogenesis. J Immunol. 1976 Oct;117(4):1325–1330. [PubMed] [Google Scholar]
- Mosher D. F. Action of fibrin-stabilizing factor on cold-insoluble globulin and alpha2-macroglobulin in clotting plasma. J Biol Chem. 1976 Mar 25;251(6):1639–1645. [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogmundsdóttir H. M., Weir D. M. Mechanisms of macrophage activation. Clin Exp Immunol. 1980 May;40(2):223–234. [PMC free article] [PubMed] [Google Scholar]
- Ojo E., Haller O., Wigzell H. Corynebacterium parvum-induced peritoneal exudate cells with rapid cytolytic activity against tumour cells are non-phagocytic cells with characteristics of natural killer cells. Scand J Immunol. 1978;8(3):215–222. doi: 10.1111/j.1365-3083.1978.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Kiessling R., Haller O. A functional comparison of tumor cell killing by activated macrophages and natural killer cells. Eur J Immunol. 1979 Apr;9(4):283–288. doi: 10.1002/eji.1830090407. [DOI] [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Schwartz R. S., Ryder R. J., Gottlieb A. A. Macrophages and antibody synthesis. Prog Allergy. 1970;14:81–144. doi: 10.1159/000289374. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Piessens W. F. Tumor cell killing by macrophages activated in vitro with lymphocyte mediators. II. Inhibition by inhibitors of protein synthesis. Cell Immunol. 1978 Jul;38(2):264–275. doi: 10.1016/0008-8749(78)90057-6. [DOI] [PubMed] [Google Scholar]
- Silkworth J. B., Loose L. D. Assessment of environmental contaminant-induced lymphocyte dysfunction. Environ Health Perspect. 1981 Jun;39:105–128. doi: 10.1289/ehp.8139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. W., Roth R., Loose L. D. A rapid, inexpensive and easily quantified assay for phagocytosis and microbicidal activity of macrophages and neutrophils. J Immunol Methods. 1979;29(3):221–226. doi: 10.1016/0022-1759(79)90309-0. [DOI] [PubMed] [Google Scholar]
- Stege T. E., Loose L. D., Di Luzio N. R. Comparative uptake of sulfobromophthalein by isolated Kupffer and parenchymal cells. Proc Soc Exp Biol Med. 1975 Jun;149(2):455–461. doi: 10.3181/00379727-149-38827. [DOI] [PubMed] [Google Scholar]
- THORBECKE G. J., BENACERRAF B. The reticulo-endothelial system and immunological phenomena. Prog Allergy. 1962;6:559–598. [PubMed] [Google Scholar]
- Tagliabue A., Mantovani A., Kilgallen M., Herberman R. B., McCoy J. L. Natural cytotoxicity of mouse monocytes and macrophages. J Immunol. 1979 Jun;122(6):2363–2370. [PubMed] [Google Scholar]
- Thigpen J. E., Faith R. E., McConnell E. E., Moore J. A. Increased susceptibility to bacterial infection as a sequela of exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Infect Immun. 1975 Dec;12(6):1319–1324. doi: 10.1128/iai.12.6.1319-1324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. G. Immune suppression as related to toxicology. CRC Crit Rev Toxicol. 1977 May;5(1):67–101. doi: 10.3109/10408447709101342. [DOI] [PubMed] [Google Scholar]
- Ward A. M., Udnoon S., Watkins J., Walker A. E., Darke C. S. Immunological mechanisms in the pathogenesis of vinyl chloride disease. Br Med J. 1976 Apr 17;1(6015):936–938. doi: 10.1136/bmj.1.6015.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Fibroblast cellular and plasma fibronectins are similar but not identical. J Cell Biol. 1979 Feb;80(2):492–498. doi: 10.1083/jcb.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]


