Abstract
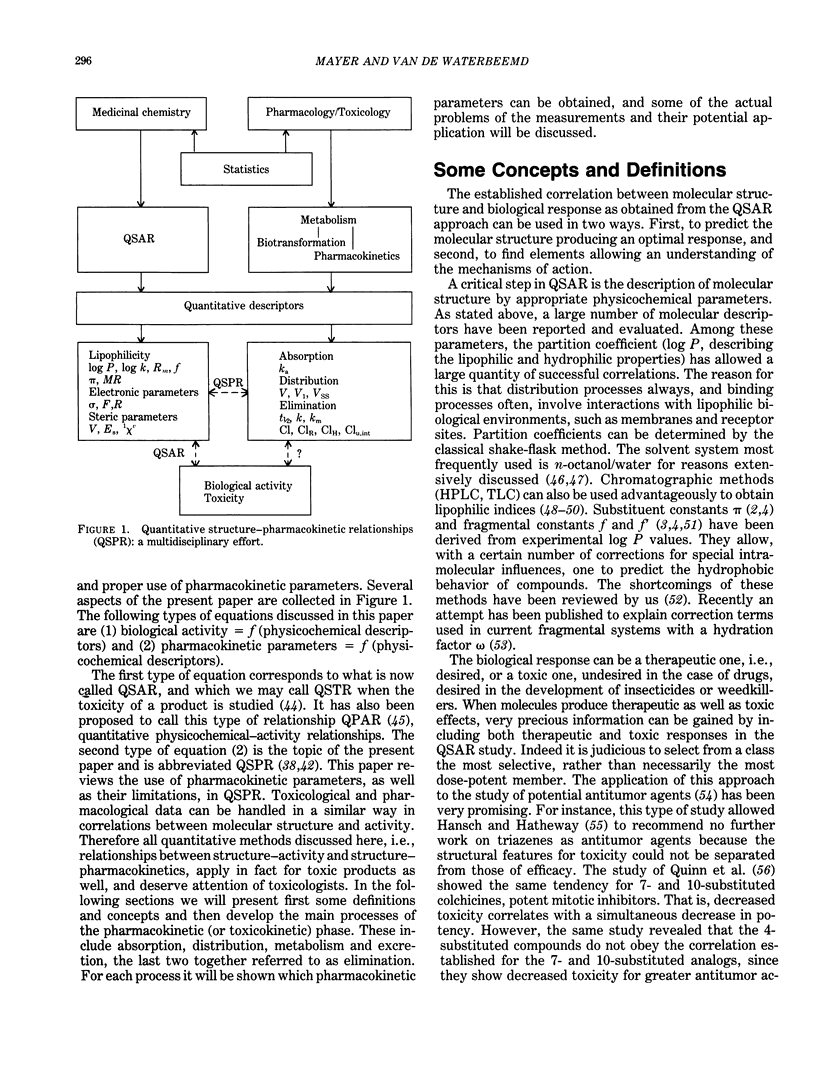
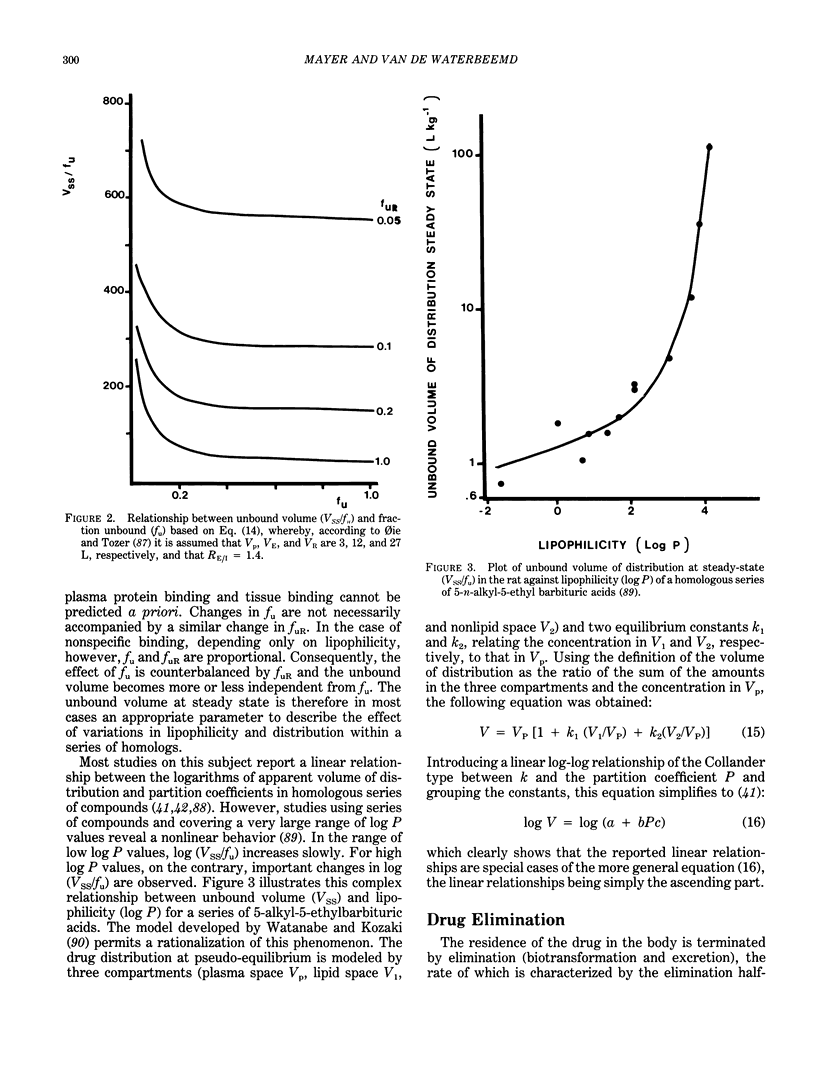
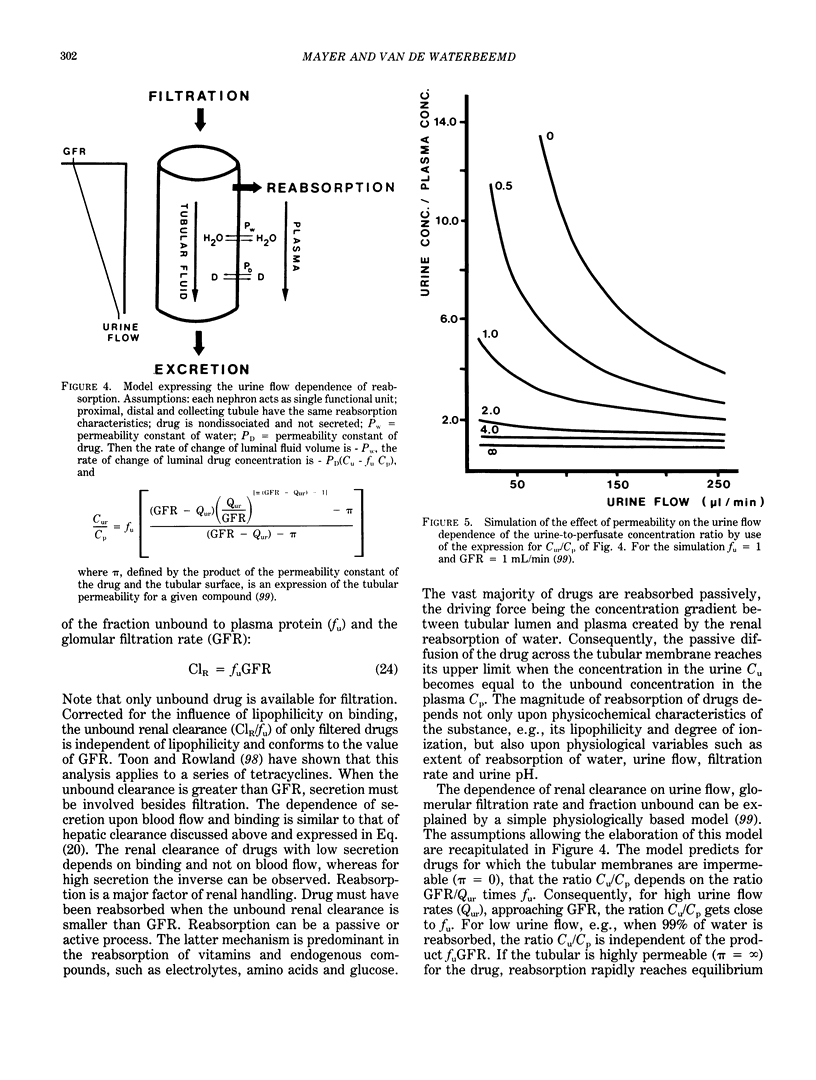
Quantitative structure-activity relationships (QSAR) relating biological activity to physiochemical descriptors have been successfully used for a number of years. It is also long recognized that pharmacokinetic parameters may play an important and even determinant role in drug action. This prompted several researchers to focus attention to pharmacokinetic parameters as potential descriptors in quantitative drug design. A number of examples of quantitative structure-pharmacokinetic relationships (QSPR) have appeared in the literature. The present contribution reviews some developments in this field. In particular, a number of concepts and problems are critically discussed, rather than compilations of examples already published in recent reviews. Attention will be paid to the main processes of the pharmacokinetic or toxicokinetic phase in drug action, including absorption, distribution and elimination (biotransformation and excretion). It is clear that quantitative approaches are of considerable interest to toxicologists, since these methods may contribute to the development of real predictive toxicology.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguiar A. J., Krc J., Jr, Kinkel A. W., Samyn J. C. Effect of polymorphism on the absorption of chloramphenicol from chloramphenicol palmitate. J Pharm Sci. 1967 Jul;56(7):847–853. doi: 10.1002/jps.2600560712. [DOI] [PubMed] [Google Scholar]
- Amidon G. L., Lee M., Lee H. Intestinal absorption of amino acid derivatives: structural requirements for membrane hydrolysis. J Pharm Sci. 1983 Aug;72(8):943–944. doi: 10.1002/jps.2600720826. [DOI] [PubMed] [Google Scholar]
- Colburn W. A. A pharmacokinetic model to differentiate preabsorptive, gut epithelial, and hepatic first-pass metabolism. J Pharmacokinet Biopharm. 1979 Aug;7(4):407–415. doi: 10.1007/BF01062538. [DOI] [PubMed] [Google Scholar]
- Colburn W. A. Simultaneous pharmacokinetic and pharmacodynamic modeling. J Pharmacokinet Biopharm. 1981 Jun;9(3):367–388. doi: 10.1007/BF01059272. [DOI] [PubMed] [Google Scholar]
- Cooper E. R., Berner B., Bruce R. D. Kinetic analysis of relationship between partition coefficient and biological response. J Pharm Sci. 1981 Jan;70(1):57–59. doi: 10.1002/jps.2600700110. [DOI] [PubMed] [Google Scholar]
- Cramer R. D., 3rd, Snader K. M., Willis C. R., Chakrin L. W., Thomas J., Sutton B. M. Application of quantitative structure-activity relationships in the development of the antiallergic pyranenamines. J Med Chem. 1979 Jun;22(6):714–725. doi: 10.1021/jm00192a019. [DOI] [PubMed] [Google Scholar]
- Cutler D. Assessment of rate and extent of drug absorption. Pharmacol Ther. 1981;14(2):123–160. doi: 10.1016/0163-7258(81)90058-9. [DOI] [PubMed] [Google Scholar]
- Denny W. A., Cain B. F., Atwell G. J., Hansch C., Panthananickal A., Leo A. Potential antitumor agents. 36. Quantitative relationships between experimental antitumor activity, toxicity, and structure for the general class of 9-anilinoacridine antitumor agents. J Med Chem. 1982 Mar;25(3):276–315. doi: 10.1021/jm00345a015. [DOI] [PubMed] [Google Scholar]
- Di Carlo F. J. Metabolism, pharmacokinetics, and toxicokinetics defined. Drug Metab Rev. 1982;13(1):1–4. doi: 10.3109/03602538209002228. [DOI] [PubMed] [Google Scholar]
- Doluisio J. T., Billups N. F., Dittert L. W., Sugita E. T., Swintosky J. V. Drug absorption. I. An in situ rat gut technique yielding realistic absorption rates. J Pharm Sci. 1969 Oct;58(10):1196–1200. doi: 10.1002/jps.2600581006. [DOI] [PubMed] [Google Scholar]
- Doluisio J. T., Crouthamel W. G., Tan G. H., Swintosky J. V., Dittert L. W. Drug absorption. 3. Effect of membrane storage on the kinetics of drug absorption. J Pharm Sci. 1970 Jan;59(1):72–76. doi: 10.1002/jps.2600590112. [DOI] [PubMed] [Google Scholar]
- Duquette P. H., Erickson R. R., Holtzman J. L. Role of substrate lipophilicity on the N-demethylation and type I binding of 3-O-alkylmorphine analogues. J Med Chem. 1983 Oct;26(10):1343–1348. doi: 10.1021/jm00364a002. [DOI] [PubMed] [Google Scholar]
- Enslein K., Craig P. N. Carcinogenesis: a predictive structure-activity model. J Toxicol Environ Health. 1982 Oct-Nov;10(4-5):521–530. doi: 10.1080/15287398209530273. [DOI] [PubMed] [Google Scholar]
- Garzia A., Villanti A., Tuccini G. Quantitative structure-selectivity relationships: selective drug design. J Pharm Sci. 1979 Sep;68(9):1081–1084. doi: 10.1002/jps.2600680905. [DOI] [PubMed] [Google Scholar]
- George C. F. Drug metabolism by the gastrointestinal mucosa. Clin Pharmacokinet. 1981 Jul-Aug;6(4):259–274. doi: 10.2165/00003088-198106040-00002. [DOI] [PubMed] [Google Scholar]
- Gerlowski L. E., Jain R. K. Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci. 1983 Oct;72(10):1103–1127. doi: 10.1002/jps.2600721003. [DOI] [PubMed] [Google Scholar]
- Hansch C., Hatheway G. J., Quinn F. R., Greenberg N. Antitumor 1-(X-aryl)-3,3-dialkyltriazenes. 2. On the role of correlation analysis in decision making in drug modification. Toxicity quantitative structure-activity relationships of 1-(X-phenyl)-3,3-dialkyltriazenes in mice. J Med Chem. 1978 Jun;21(6):574–577. doi: 10.1021/jm00204a013. [DOI] [PubMed] [Google Scholar]
- Kapetanović I. M., Strong J. M., Mieyal J. J. Metabolic structure-activity relationship for a homologous series of phenacetin analogs. J Pharmacol Exp Ther. 1979 Apr;209(1):20–24. [PubMed] [Google Scholar]
- Kubinyi H. Lipophilicity and drug activity. Prog Drug Res. 1979;23:97–198. doi: 10.1007/978-3-0348-7105-1_5. [DOI] [PubMed] [Google Scholar]
- Nimmo W. S. Drugs, diseases and altered gastric emptying. Clin Pharmacokinet. 1976;1(3):189–203. doi: 10.2165/00003088-197601030-00002. [DOI] [PubMed] [Google Scholar]
- Norrington F. E., Hyde R. M., Williams S. G., Wootton R. Physiochemical-activity relations in practice. 1. A rational and self-consistent data bank. J Med Chem. 1975 Jun;18(6):604–607. doi: 10.1021/jm00240a016. [DOI] [PubMed] [Google Scholar]
- Notari R. E. Prodrug design. Pharmacol Ther. 1981;14(1):25–53. doi: 10.1016/0163-7258(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Oie S., Tozer T. N. Effect of altered plasma protein binding on apparent volume of distribution. J Pharm Sci. 1979 Sep;68(9):1203–1205. doi: 10.1002/jps.2600680948. [DOI] [PubMed] [Google Scholar]
- Pang K. S., Rowland M. Hepatic clearance of drugs. I. Theoretical considerations of a "well-stirred" model and a "parallel tube" model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J Pharmacokinet Biopharm. 1977 Dec;5(6):625–653. doi: 10.1007/BF01059688. [DOI] [PubMed] [Google Scholar]
- Plá-Delfina J. M., Moreno J. Intestinal absorption-partition relationships: a tentative functional nonlinear model. J Pharmacokinet Biopharm. 1981 Apr;9(2):191–215. doi: 10.1007/BF01068082. [DOI] [PubMed] [Google Scholar]
- Quinn F. R., Neiman Z., Beisler J. A. Toxicity quantitative structure--activity relationships of colchicines. J Med Chem. 1981 May;24(5):636–639. doi: 10.1021/jm00137a031. [DOI] [PubMed] [Google Scholar]
- Ritschel W. A., Hammer G. V. Prediction of the volume of distribution from in vitro data and use for estimating the absolute extent of absorption. Int J Clin Pharmacol Ther Toxicol. 1980 Jul;18(7):298–316. [PubMed] [Google Scholar]
- Rowland M., Benet L. Z., Graham G. G. Clearance concepts in pharmacokinetics. J Pharmacokinet Biopharm. 1973 Apr;1(2):123–136. doi: 10.1007/BF01059626. [DOI] [PubMed] [Google Scholar]
- Rowland M. Influence of route of administration on drug availability. J Pharm Sci. 1972 Jan;61(1):70–74. doi: 10.1002/jps.2600610111. [DOI] [PubMed] [Google Scholar]
- Rowland M., Tucker G. Symbols in pharmacokinetics. J Pharmacokinet Biopharm. 1980 Oct;8(5):497–507. doi: 10.1007/BF01059548. [DOI] [PubMed] [Google Scholar]
- Sargent N. S., Wood S. G., Upshall D. G., Bridges J. W. The relationship between chemical structure and the in vivo metabolism of an homologous series of n-alkyl carbamates. J Pharm Pharmacol. 1982 Jun;34(6):367–372. doi: 10.1111/j.2042-7158.1982.tb04731.x. [DOI] [PubMed] [Google Scholar]
- Scholtan W. Die hydrophobe bindung der Pharmaka an Humanalbumin und Ribonucleinsäure. Arzneimittelforschung. 1968 May;18(5):505–517. [PubMed] [Google Scholar]
- Schultz T. W., Cajina-Quezada M. Structure-toxicity relationships of selected nitrogenous heterocyclic compounds II. Dinitrogen molecules. Arch Environ Contam Toxicol. 1982;11(3):353–361. doi: 10.1007/BF01055212. [DOI] [PubMed] [Google Scholar]
- Seydel J. K., Schaper K. J. Quantitative structure-pharmacokinetic relationships and drug design. Pharmacol Ther. 1981;15(2):131–182. doi: 10.1016/0163-7258(81)90040-1. [DOI] [PubMed] [Google Scholar]
- Smith R. N., Hansch C., Ames M. M. Selection of a reference partitioning system for drug design work. J Pharm Sci. 1975 Apr;64(4):599–606. doi: 10.1002/jps.2600640405. [DOI] [PubMed] [Google Scholar]
- Sudlow G., Birkett D. J., Wade D. N. Further characterization of specific drug binding sites on human serum albumin. Mol Pharmacol. 1976 Nov;12(6):1052–1061. [PubMed] [Google Scholar]
- Testa B., Salvesen B. Quantitative structure-activity relationships in drug metabolism and disposition: pharmacokinetics of N-substituted amphetamines in humans. J Pharm Sci. 1980 May;69(5):497–501. doi: 10.1002/jps.2600690505. [DOI] [PubMed] [Google Scholar]
- Tomlinson E. Chromatographic hydrophobic parameters in correlation analysis of structure-activity relationships. J Chromatogr. 1975 Mar 12;113(1):1–45. doi: 10.1016/s0021-9673(00)88797-x. [DOI] [PubMed] [Google Scholar]
- Toon S., Rowland M. Structure-pharmacokinetic relationships among the barbiturates in the rat. J Pharmacol Exp Ther. 1983 Jun;225(3):752–763. [PubMed] [Google Scholar]
- Tucker G. T. Measurement of the renal clearance of drugs. Br J Clin Pharmacol. 1981 Dec;12(6):761–770. doi: 10.1111/j.1365-2125.1981.tb01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallner J. J. Binding of drugs by albumin and plasma protein. J Pharm Sci. 1977 Apr;66(4):447–465. doi: 10.1002/jps.2600660402. [DOI] [PubMed] [Google Scholar]
- Watanabe J., Kozaki A. Relationship between partition coefficients and apparent volumes of distribution for basic drugs. II. Chem Pharm Bull (Tokyo) 1978 Nov;26(11):3463–3470. doi: 10.1248/cpb.26.3463. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. R., Shand D. G. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975 Oct;18(4):377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]
- Winne D. Influence of blood flow on intestinal absorption of xenobiotics. Pharmacology. 1980;21(1):1–15. doi: 10.1159/000137409. [DOI] [PubMed] [Google Scholar]
- Wishnok J. S., Archer M. C., Edelman A. S., Rand W. M. Nitrosamine carcinogenicity: a quantitative Hansch-Taft structure-activity relationship. Chem Biol Interact. 1978 Jan;20(1):43–54. doi: 10.1016/0009-2797(78)90079-0. [DOI] [PubMed] [Google Scholar]
- Workman P., Brown J. M. Structure-pharmacokinetic relationships for misonidazole analogues in mice. Cancer Chemother Pharmacol. 1981;6(1):39–49. doi: 10.1007/BF00253009. [DOI] [PubMed] [Google Scholar]



