Abstract
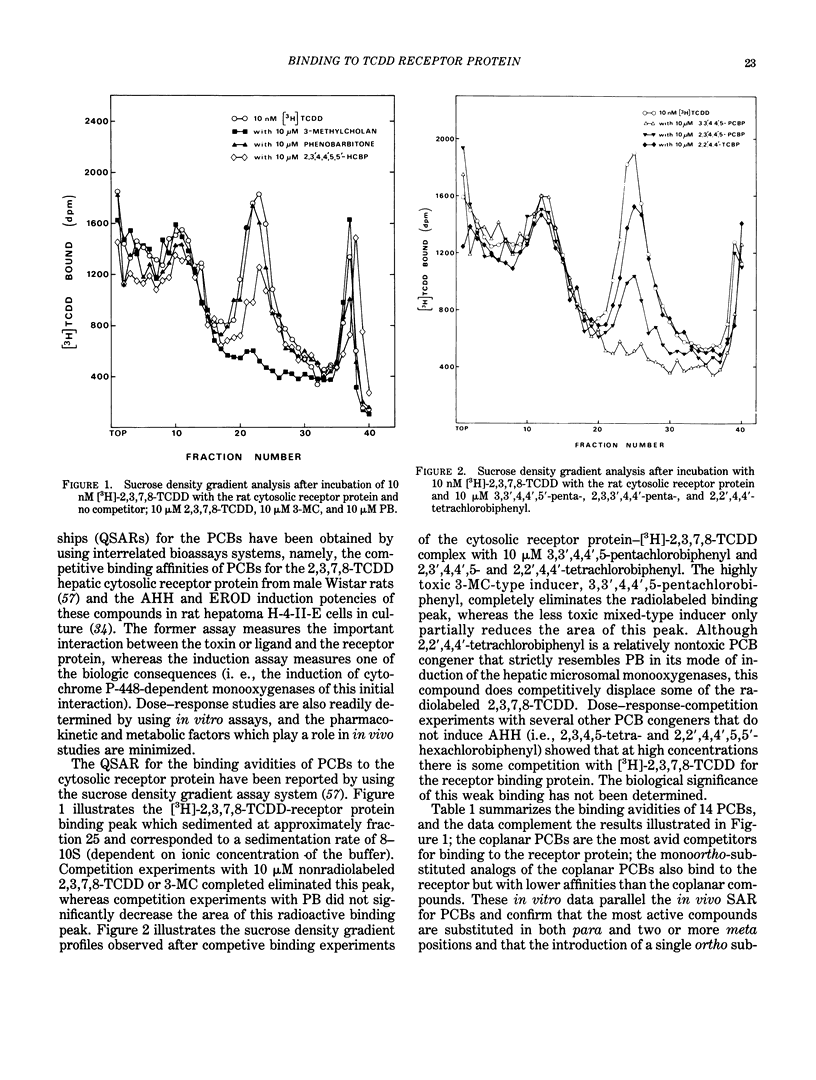
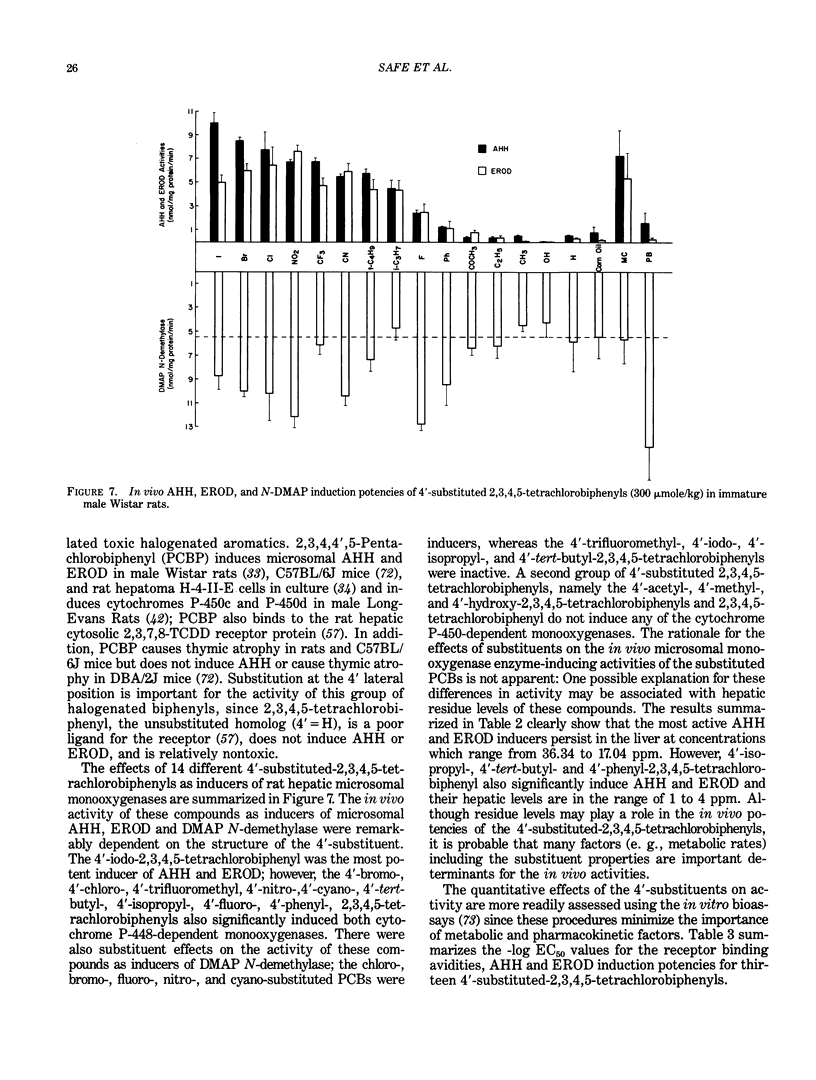
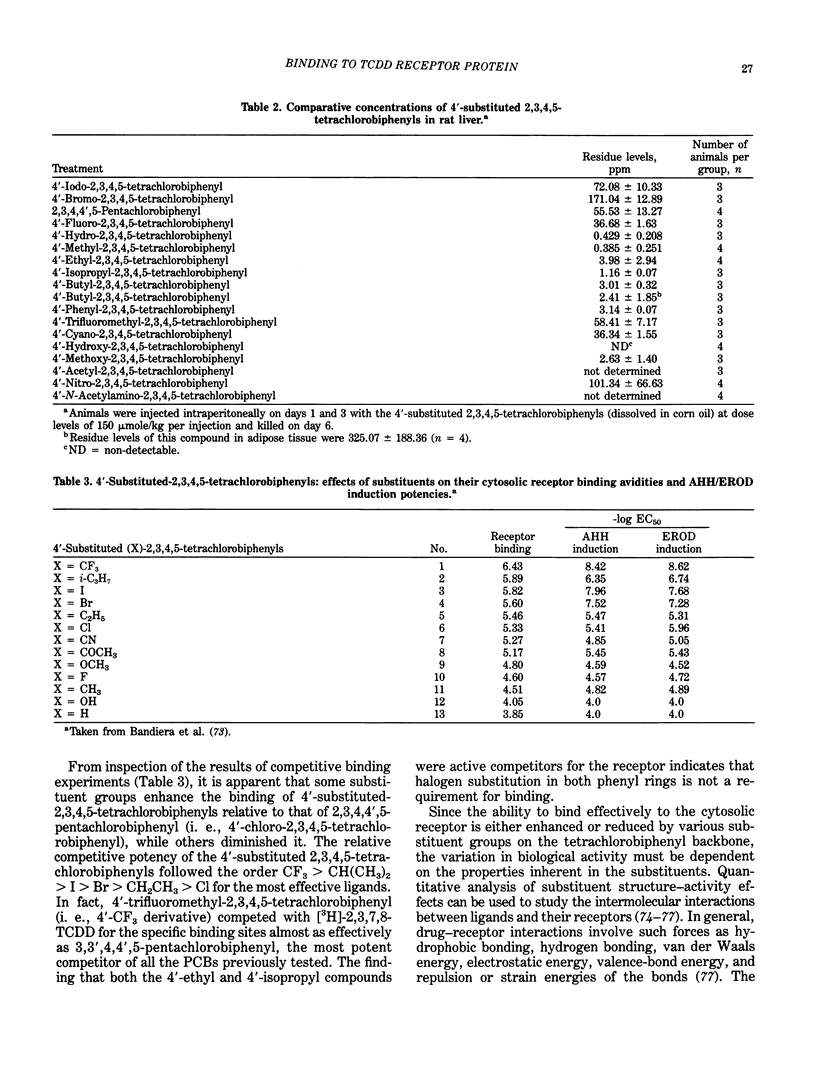
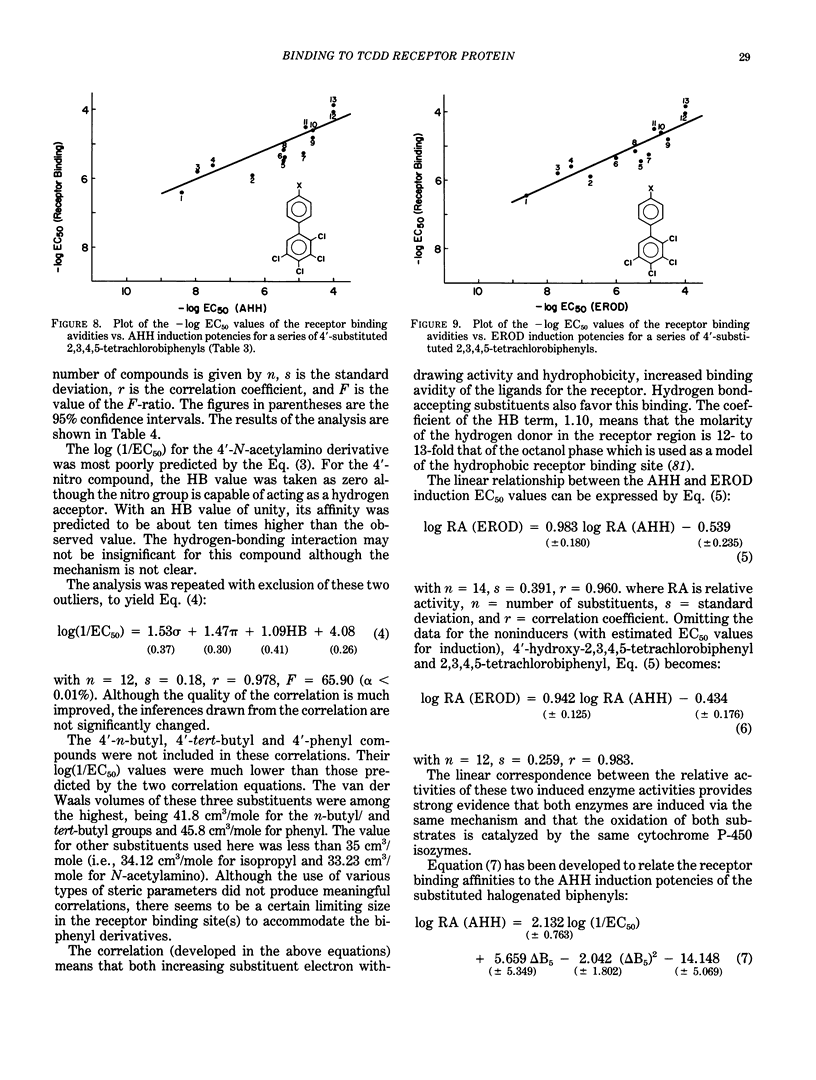
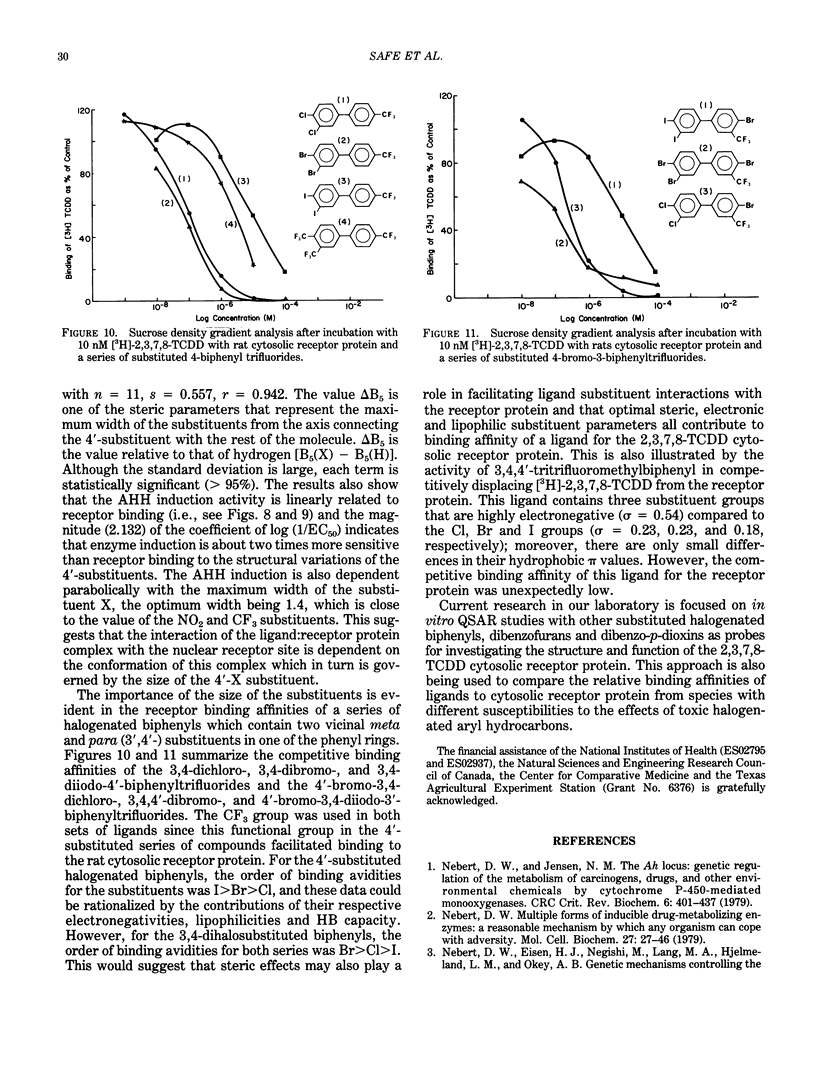
The quantitative structure-activity relationships (QSARs) for polychlorinated biphenyl (PCB) congeners have been determined by comparing the EC50 values for three in vitro test systems, namely, aryl hydrocarbon hydroxylase (AHH) and ethoxyresorufin O-deethylase (EROD) induction in rat hepatoma H-4-II-E cells and competitive binding avidities to the rat cytosolic receptor protein (using 2,3,7,8-tetrachlorodibenzo-p-dioxin as a radioligand). For several PCB congeners that are in vivo inducers of rat hepatic microsomal AHH, there was a linear correlation between the -log EC50 values for receptor and the -log EC50 values for AHH (or EROD) induction; moreover, a comparable linear relationship was observed between the -log EC50 values for AHH and EROD induction. Previous in vivo studies have shown that the most active PCB congeners 3,3',4,4'-tetra-, 3,4,4',5-tetra-, 3,3',4,4',5-penta-, and 3,3',4,4',5,5'-hexachlorobiphenyl, cause many of the biologic and toxic effects reported for the highly toxic halogenated aryl hydrocarbon, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Moreover, the monoortho-substituted homologs of the four coplanar PCBs also elicit comparable in vivo biologic and toxic responses. It was evident from the QSARs for PCBs that there was an excellent correspondence between the in vivo and in vitro potencies of the individual PCB congeners. The effects of substituents on both receptor binding and AHH/EROD induction was determined for a series of 4'-substituted (X)-2,3,4,5-tetrachlorobiphenyls (where X = H, Cl, Br, I, OH, OCH3, NO2, COCH3, F, CF3, CH3, C2H5, i-C3H7, n-C4H9 and t-C4H9). Not unexpectedly, there was a linear relationship between the -log EC50 values for AHH and EROD induction, and these results confirm that both enzymatic oxidations are catalyzed by the same cytochrome P-450 isozyme(s). The effects of substituent structure on receptor binding for 12 substituents was subjected to multiple regression analysis which correlates the relative binding affinities of the compounds with the physical chemical characteristics of the substituents. The analysis gave the following equation: log (1/EC50) = 1.53 sigma + 1.47 pi + 1.09 HB + 4.08 for n = 12, s = 0.18, r = 0.978; where n is the number of substituents, s is the standard deviation, r is the correlation coefficient, and sigma = electronegativity, pi = hydrophobicity (log P) and HB = hydrogen bonding capacity for the substituent groups.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF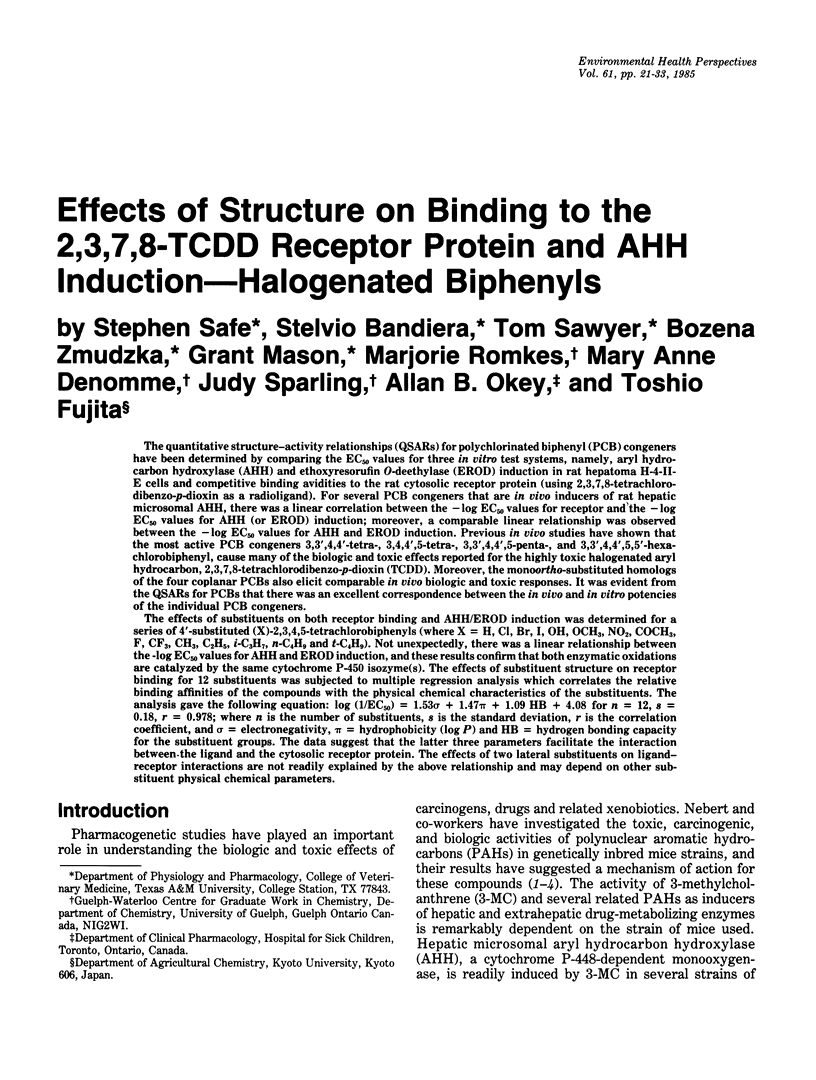





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albro P. W., McKinney J. D. The relationship between polarizability of polychlorinated biphenyls and their induction of mixed function oxidase activity. Chem Biol Interact. 1981 Mar 15;34(3):373–378. doi: 10.1016/0009-2797(81)90109-5. [DOI] [PubMed] [Google Scholar]
- Andres J., Lambert I., Robertson L., Bandiera S., Sawyer T., Lovering S., Safe S. The comparative biologic and toxic potencies of polychlorinated biphenyls and polybrominated biphenyls. Toxicol Appl Pharmacol. 1983 Sep 15;70(2):204–215. doi: 10.1016/0041-008x(83)90096-0. [DOI] [PubMed] [Google Scholar]
- Ax R. L., Hansen L. G. Effects of purified polychlorinated biphenyl analogs on chicken reproduction. Poult Sci. 1975 May;54(3):895–900. doi: 10.3382/ps.0540895. [DOI] [PubMed] [Google Scholar]
- Bandiera S., Safe S., Okey A. B. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982 Apr;39(3):259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Bandiera S., Sawyer T. W., Campbell M. A., Fujita T., Safe S. Competitive binding to the cytosolic 2,3,7,8-tetrachlorodibenzo-p-dioxin receptor. Effects of structure on the affinities of substituted halogenated biphenyls--a QSAR analysis. Biochem Pharmacol. 1983 Dec 15;32(24):3803–3813. doi: 10.1016/0006-2952(83)90153-3. [DOI] [PubMed] [Google Scholar]
- Bandiera S., Sawyer T., Campbell M. A., Robertson L., Safe S. Halogenated biphenyls as AHH inducers: effects of different halogen substituents. Life Sci. 1982 Aug 9;31(6):517–525. doi: 10.1016/0024-3205(82)90479-9. [DOI] [PubMed] [Google Scholar]
- Benedict W. F., Considine N., Nebert D. W. Genetic differences in aryl hydrocarbon hydroxylase induction and benzo(a)pyrene-produced tumorigenesis in the mouse. Mol Pharmacol. 1973 Mar;9(2):266–277. [PubMed] [Google Scholar]
- Biocca M., Gupta B. N., Chae K., McKinney J. D., Moore J. A. Toxicity of selected symmetrical hexachlorobiphenyl isomers in the mouse. Toxicol Appl Pharmacol. 1981 May;58(3):461–474. doi: 10.1016/0041-008x(81)90099-5. [DOI] [PubMed] [Google Scholar]
- Bradlaw J. A., Garthoff L. H., Hurley N. E., Firestone D. Comparative induction of aryl hydrocarbon hydroxylase activity in vitro by analogues of dibenzo-p-dioxin. Food Cosmet Toxicol. 1980 Dec;18(6):627–635. doi: 10.1016/s0015-6264(80)80011-3. [DOI] [PubMed] [Google Scholar]
- Dannan G. A., Guengerich F. P., Kaminsky L. S., Aust S. D. Regulation of cytochrome P-450. Immunochemical quantitation of eight isozymes in liver microsomes of rats treated with polybrominated biphenyl congeners. J Biol Chem. 1983 Jan 25;258(2):1282–1288. [PubMed] [Google Scholar]
- Duran-Reynals M. L., Lilly F., Bosch A., Blank K. J. The genetic basis of susceptibility to leukemia induction in mice by 3-methylcholanthrene applied percutaneously. J Exp Med. 1978 Feb 1;147(2):459–469. doi: 10.1084/jem.147.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T. Analysis and prediction of partition coefficients of meta- and para-disubstituted benzenes in terms of substituent effects. J Pharm Sci. 1983 Mar;72(3):285–289. doi: 10.1002/jps.2600720319. [DOI] [PubMed] [Google Scholar]
- Fujita T., Nishioka T., Nakajima M. Hydrogen-bonding parameter and its significance in quantitative structure--activity studies. J Med Chem. 1977 Aug;20(8):1071–1081. doi: 10.1021/jm00218a017. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Neal R. A. The examination and quantitation of tissue cytosolic receptors for 2,3,7,8-tetrachlorodibenzo-p-dioxin using hydroxylapatite. Anal Biochem. 1982 Jul 15;124(1):1–11. doi: 10.1016/0003-2697(82)90212-3. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A., Hickman P., Bergman H., McKinney J. D., Walker M. P. Separation of pure polychlorinated biphenyl isomers into two types of inducers on the basis of induction of cytochrome P-450 or P-448. Chem Biol Interact. 1977 Apr;17(1):69–87. doi: 10.1016/0009-2797(77)90073-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A., Linko P., Luster M. I., Sundheimer D. W. Purification and characterization of a second form of hepatic cytochrome P-448 from rats treated with a pure polychlorinated biphenyl isomer. J Biol Chem. 1982 Mar 10;257(5):2702–2707. [PubMed] [Google Scholar]
- Goldstein J. A., McKinney J. D., Lucier G. W., Hickman P., Bergman H., Moore J. A. Toxicological assessment of hexachlorobiphenyl isomers and 2,3,7,8,-tetrachlorodibenzofuran in chicks. II. Effects on drug metabolism and porphyrin accumulation. Toxicol Appl Pharmacol. 1976 Apr;36(1):81–92. doi: 10.1016/0041-008x(76)90028-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. A. The structure-activity relationships of halogenated biphenyls as enzyme inducers. Ann N Y Acad Sci. 1979 May 31;320:164–178. [PubMed] [Google Scholar]
- HANSCH C., DEUTSCH E. W., SMITH R. N. THE USE OF SUBSTITUENT CONSTANTS AND REGRESSION ANALYSIS IN THE STUDY OF ENZYMATIC REACTION MECHANISMS. J Am Chem Soc. 1965 Jun 20;87:2738–2742. doi: 10.1021/ja01090a035. [DOI] [PubMed] [Google Scholar]
- Hansch C., Leo A., Unger S. H., Kim K. H., Nikaitani D., Lien E. J. "Aromatic" substituent constants for structure-activity correlations. J Med Chem. 1973 Nov;16(11):1207–1216. doi: 10.1021/jm00269a003. [DOI] [PubMed] [Google Scholar]
- Jones K. G., Sweeney G. D. Dependence of the porphyrogenic effect of 2,3,7,8-tetrachlorodibenzo(p)dioxin upon inheritance of aryl hydrocarbon hydroxylase responsiveness. Toxicol Appl Pharmacol. 1980 Mar 30;53(1):42–49. doi: 10.1016/0041-008x(80)90379-8. [DOI] [PubMed] [Google Scholar]
- Kohli K. K., Philpot R. M., Albro P. W., McKinney J. D. Induction of different species of cytochrome P-450 by coplanar and noncoplanar isomers of hexachlorobiphenyl. Life Sci. 1980 Mar 24;26(12):945–952. doi: 10.1016/0024-3205(80)90115-0. [DOI] [PubMed] [Google Scholar]
- Kouri R. E., Ratrie H., 3rd, Whitmire C. E. Genetic control of susceptibility to 3-methylcholanthrene-induced subcutaneous sarcomas. Int J Cancer. 1974 May 15;13(5):714–720. doi: 10.1002/ijc.2910130515. [DOI] [PubMed] [Google Scholar]
- Legraverend C., Mansour B., Nebert D. W., Holland J. M. Genetic differences in benzo[a]pyrene-initiated tumorigenesis in mouse skin. Pharmacology. 1980;20(5):242–255. doi: 10.1159/000137370. [DOI] [PubMed] [Google Scholar]
- Marks T. A., Kimmel G. L., Staples R. E. Influence of symmetrical polychlorinated biphenyl isomers on embryo and fetal development in mice. I. Teratogenicity of 3, 3', 4, 4', 5, 5',-hexachlorobiphenyl. Toxicol Appl Pharmacol. 1981 Nov;61(2):269–276. doi: 10.1016/0041-008x(81)90417-8. [DOI] [PubMed] [Google Scholar]
- Mason M. E., Okey A. B. Cytosolic and nuclear binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to the Ah receptor in extra-hepatic tissues of rats and mice. Eur J Biochem. 1982 Mar;123(1):209–215. doi: 10.1111/j.1432-1033.1982.tb06518.x. [DOI] [PubMed] [Google Scholar]
- Mattison D. R., Thorgeirsson S. S. Ovarian aryl hydrocarbon hydroxylase activity and primordial oocyte toxicity of polycyclic aromatic hydrocarbons in mice. Cancer Res. 1979 Sep;39(9):3471–3475. [PubMed] [Google Scholar]
- McKinney J. D., Chae K., Gupta B. N., Moore J. A., Goldstein H. A. Toxicological assessment of hexachlorobiphenyl isomers and 2,3,7,8 tetrachlorodibenzofuran in chicks. I. Relationship of chemical parameters. Toxicol Appl Pharmacol. 1976 Apr;36(1):65–80. doi: 10.1016/0041-008x(76)90027-2. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Singh P. Structure-activity relationships in halogenated biphenyls: unifying hypothesis for structural specificity. Chem Biol Interact. 1981 Jan;33(2-3):271–283. doi: 10.1016/0009-2797(81)90046-6. [DOI] [PubMed] [Google Scholar]
- Nagayama J., Kuroki H., Masuda Y., Kuratsune M. A comparative study of polychlorinated dibenzofurans, polychlorinated biphenyls and 2,3,7,8-tetrachlorodibenzo-p-dioxin on aryl hydrocarbon hydroxylase inducing potency in rats. Arch Toxicol. 1983 Jul;53(3):177–184. doi: 10.1007/BF00316501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Eisen H. J., Negishi M., Lang M. A., Hjelmeland L. M., Okey A. B. Genetic mechanisms controlling the induction of polysubstrate monooxygenase (P-450) activities. Annu Rev Pharmacol Toxicol. 1981;21:431–462. doi: 10.1146/annurev.pa.21.040181.002243. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jensen N. M. The Ah locus: genetic regulation of the metabolism of carcinogens, drugs, and other environmental chemicals by cytochrome P-450-mediated monooxygenases. CRC Crit Rev Biochem. 1979;6(4):401–437. doi: 10.3109/10409237909105427. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Multiple forms of inducible drug-metabolizing enzymes: a reasonable mechanism by which any organism can cope with adversity. Mol Cell Biochem. 1979 Sep 28;27(1):27–46. doi: 10.1007/BF00849277. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Robinson J. R., Niwa A., Kumaki K., Poland A. P. Genetic expression of aryl hydrocarbon hydroxylase activity in the mouse. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):393–414. doi: 10.1002/jcp.1040850407. [DOI] [PubMed] [Google Scholar]
- Oishi S., Morita M., Fukuda H. Comparative toxicity of polychlorinated biphenyls and dibenzofurans in rats. Toxicol Appl Pharmacol. 1978 Jan;43(1):13–22. doi: 10.1016/s0041-008x(78)80028-3. [DOI] [PubMed] [Google Scholar]
- Okey A. B., Vella L. M. Binding of 3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin to a common Ah receptor site in mouse and rat hepatic cytosols. Eur J Biochem. 1982 Sep;127(1):39–47. doi: 10.1111/j.1432-1033.1982.tb06834.x. [DOI] [PubMed] [Google Scholar]
- Ozawa N., Yoshihara S., Kawano K., Okada Y., Yoshimura H. 3,4,5,3',4'-Pentachlorobiphenyl as a useful inducer for purification of rat liver microsomal cytochrome P448. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1140–1147. doi: 10.1016/0006-291x(79)91999-5. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Cockerline R., Safe S. Induction of both 3-methylcholanthrene- and phenobarbitone-type microsomal enzyme activity by a single polychlorinated biphenyl isomer. Biochem Pharmacol. 1980 Feb;29(2):259–262. doi: 10.1016/0006-2952(80)90339-1. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Cockerline R., Safe S. Polychlorinated biphenyl isomers and congeners as inducers of both 3-methylcholanthrene- and phenobarbitone-type microsomal enzyme activity. Chem Biol Interact. 1980 Mar;29(3):277–289. doi: 10.1016/0009-2797(80)90147-7. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Robertson L., Safe L., Safe S. Polychlorinated biphenyls as inducers of hepatic microsomal enzymes: structure-activity rules. Chem Biol Interact. 1980 Jun;30(3):271–285. doi: 10.1016/0009-2797(80)90050-2. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Robertson L., Uhlig L., Campbell M. A., Safe S. 2,3,4,4'-Pentachlorobiphenyl: differential effects on C57BL/6J and DBA/2J inbred mice. Biochem Pharmacol. 1982 Sep 1;31(17):2830–2833. doi: 10.1016/0006-2952(82)90143-5. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Safe S. H., Robertson L. W., Thomas P. E., Ryan D. E., Reik L. M., Levin W. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphenyl-treated rats. A study of structure-activity relationships. J Biol Chem. 1983 May 10;258(9):5967–5976. [PubMed] [Google Scholar]
- Poland A. P., Glover E., Robinson J. R., Nebert D. W. Genetic expression of aryl hydrocarbon hydroxylase activity. Induction of monooxygenase activities and cytochrome P1-450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically "nonresponsive" to other aromatic hydrocarbons. J Biol Chem. 1974 Sep 10;249(17):5599–5606. [PubMed] [Google Scholar]
- Poland A., Glover E. 2,3,7,8,-Tetrachlorodibenzo-p-dioxin: segregation of toxocity with the Ah locus. Mol Pharmacol. 1980 Jan;17(1):86–94. [PubMed] [Google Scholar]
- Poland A., Glover E. Chlorinated biphenyl induction of aryl hydrocarbon hydroxylase activity: a study of the structure-activity relationship. Mol Pharmacol. 1977 Sep;13(5):924–938. [PubMed] [Google Scholar]
- Poland A., Glover E. Chlorinated dibenzo-p-dioxins: potent inducers of delta-aminolevulinic acid synthetase and aryl hydrocarbon hydroxylase. II. A study of the structure-activity relationship. Mol Pharmacol. 1973 Nov;9(6):736–747. [PubMed] [Google Scholar]
- Poland A., Greenlee W. F., Kende A. S. Studies on the mechanism of action of the chlorinated dibenzo-p-dioxins and related compounds. Ann N Y Acad Sci. 1979 May 31;320:214–230. doi: 10.1111/j.1749-6632.1979.tb56603.x. [DOI] [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Puhvel S. M., Sakamoto M., Ertl D. C., Reisner R. M. Hairless mice as models for chloracne: a study of cutaneous changes induced by topical application of established chloracnegens. Toxicol Appl Pharmacol. 1982 Jul;64(3):492–503. doi: 10.1016/0041-008x(82)90247-2. [DOI] [PubMed] [Google Scholar]
- Render J. A., Aust S. D., Sleight S. D. Acute pathologic effects of 3,3',4,4',5,5'-hexabromobiphenyl in rats: comparison of its effects with Firemaster BP-6 and 2,2',4,4',5,5'-hexabromobiphenyl. Toxicol Appl Pharmacol. 1982 Mar 15;62(3):428–444. doi: 10.1016/0041-008x(82)90144-2. [DOI] [PubMed] [Google Scholar]
- Robertson L. W., Parkinson A., Bandiera S., Lambert I., Merrill J., Safe S. H. PCBs and PBBs: biologic and toxic effects on C57BL/6J and DBA/2J inbred mice. Toxicology. 1984 Jun;31(3-4):191–206. doi: 10.1016/0300-483x(84)90101-x. [DOI] [PubMed] [Google Scholar]
- Robertson L. W., Parkinson A., Bandiera S., Safe S. Potent induction of rat liver microsomal, drug-metabolizing enzymes by 2,3,3',4,4',5-hexabromobiphenyl, a component of fireMaster. Chem Biol Interact. 1981 Apr;35(1):13–24. doi: 10.1016/0009-2797(81)90060-0. [DOI] [PubMed] [Google Scholar]
- Robertson L. W., Parkinson A., Campbell M. A., Safe S. Polybrominated biphenyls as aryl hydrocarbon hydroxylase inducers: structure-activity correlations. Chem Biol Interact. 1982 Oct;42(1):53–66. doi: 10.1016/0009-2797(82)90141-7. [DOI] [PubMed] [Google Scholar]
- Robertson L. W., Parkinson A., Safe S. Induction of both cytochromes P-450 and P-448 by 2,3',4,4',5-pentabromobiphenyl, a component of fireMaster. Biochem Biophys Res Commun. 1980 Jan 15;92(1):175–182. doi: 10.1016/0006-291x(80)91536-3. [DOI] [PubMed] [Google Scholar]
- Robinson J. R., Felton J. S., Levitt R. C., Thorgeirsson S. S., Nebert D. W. Relationship between "aromatic hydrocarbon responsiveness" and the survival times in mice treated with various drugs and environmental compounds. Mol Pharmacol. 1975 Nov;11(6):850–865. [PubMed] [Google Scholar]
- Safe S., Robertson L. W., Safe L., Parkinson A., Bandiera S., Sawyer T., Campbell M. A. Halogenated biphenyls: molecular toxicology. Can J Physiol Pharmacol. 1982 Jul;60(7):1057–1064. doi: 10.1139/y82-151. [DOI] [PubMed] [Google Scholar]
- Shum S., Jensen N. M., Nebert D. W. The murine Ah locus: in utero toxicity and teratogenesis associated with genetic differences in benzo[a]pyrene metabolism. Teratology. 1979 Dec;20(3):365–376. doi: 10.1002/tera.1420200307. [DOI] [PubMed] [Google Scholar]
- Silkworth J. B., Grabstein E. M. Polychlorinated biphenyl immunotoxicity: dependence on isomer planarity and the Ah gene complex. Toxicol Appl Pharmacol. 1982 Aug;65(1):109–115. doi: 10.1016/0041-008x(82)90368-4. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Hutton J. J., Taylor B. A. Genetic relationship between aryl hydrocarbon hydroxylase inducibility and chemical carcinogen induced skin ulceration in mice. Genetics. 1973 Aug;74(4):655–659. doi: 10.1093/genetics/74.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey R. H., Hannah R. R., Negishi M., Nebert D. W., Eisen H. J. The Ah locus: correlation of intranuclear appearance of inducer-receptor complex with induction of cytochrome P1-450 mRNA. Cell. 1982 Nov;31(1):275–284. doi: 10.1016/0092-8674(82)90427-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. A., Yoshimura H., Fujita M., Yamamoto T. Metabolic and toxicologic evaluation of 2,34,3',4'-pentachlorobiphenyl in rats and mice. Chem Pharm Bull (Tokyo) 1976 Sep;24(9):2168–2174. doi: 10.1248/cpb.24.2168. [DOI] [PubMed] [Google Scholar]
- Yoshihara S., Nagata K., Yoshimura H., Kuroki H., Masuda Y. Inductive effect on hepatic enzymes and acute toxicity of individual polychlorinated dibenzofuran congeners in rats. Toxicol Appl Pharmacol. 1981 Jul;59(3):580–588. doi: 10.1016/0041-008x(81)90313-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Yoshihara S., Ozawa N., Miki M. Possible correlation between induction modes of hepatic enzymes by PCBs and their toxicity in rats. Ann N Y Acad Sci. 1979 May 31;320:179–192. doi: 10.1111/j.1749-6632.1979.tb56600.x. [DOI] [PubMed] [Google Scholar]



