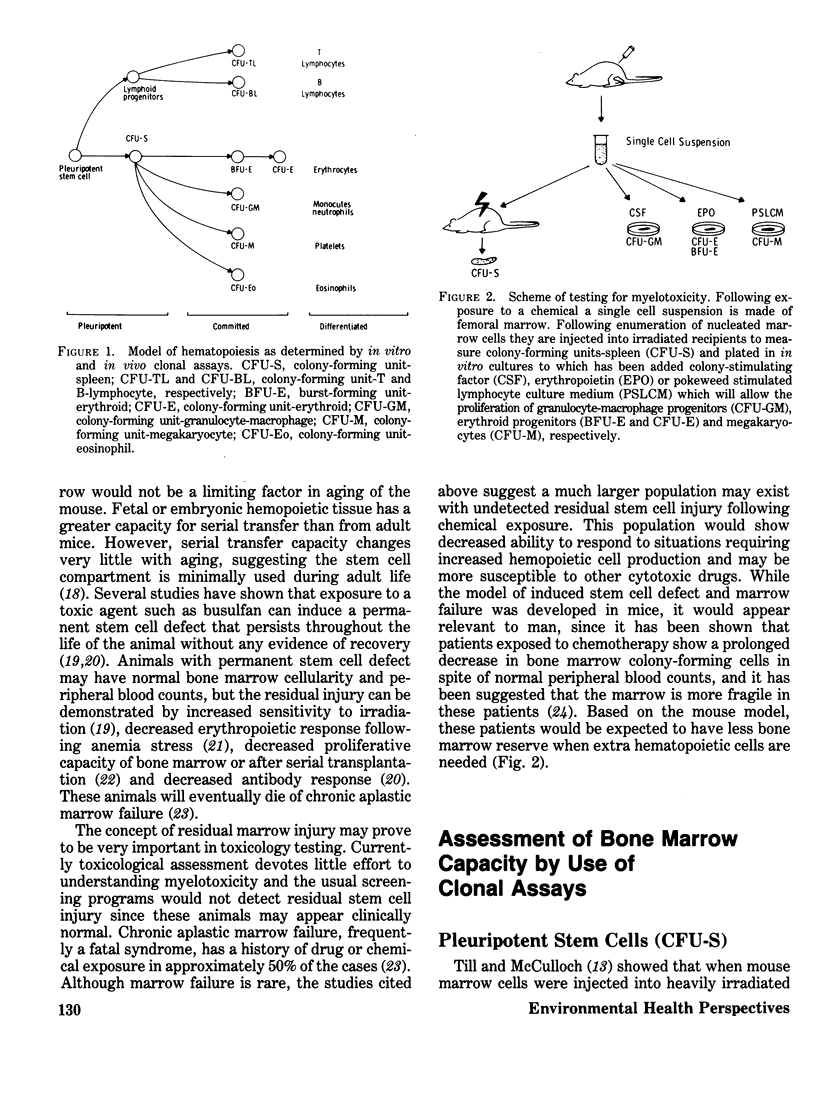
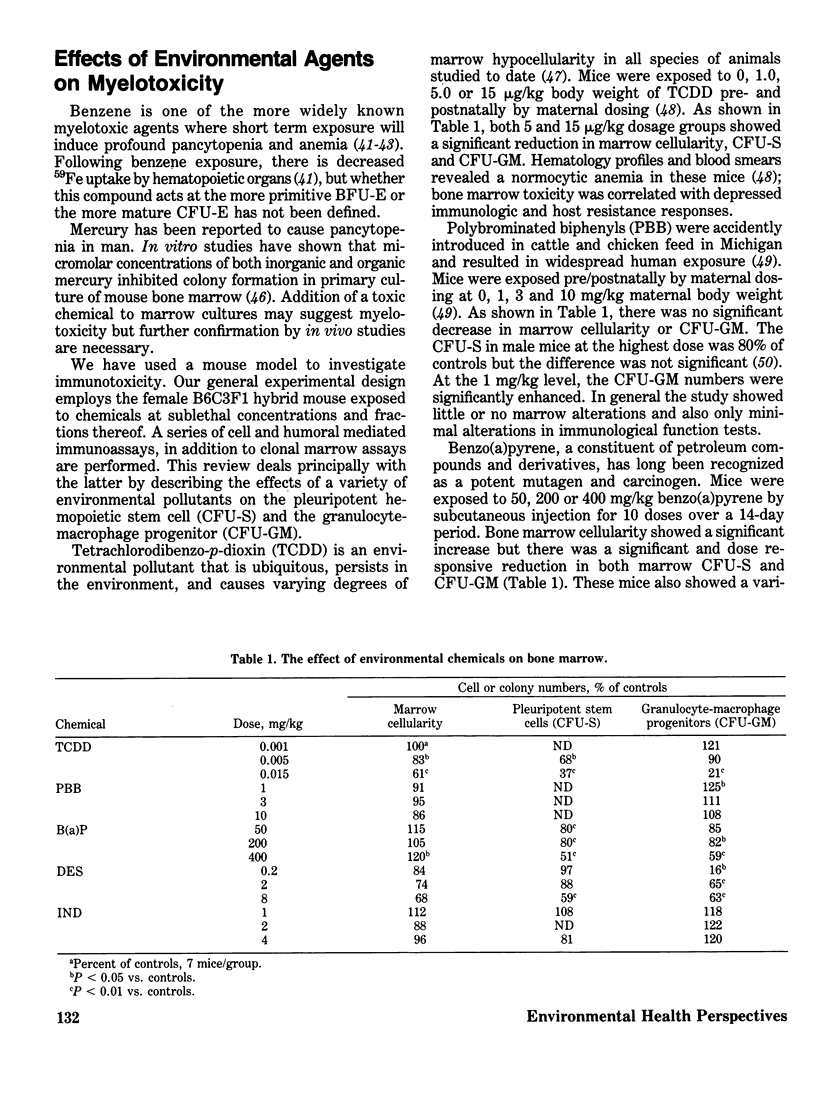
Abstract
Potential antineoplastic agents must be screened for the delayed toxicity that occurs in many cases of drug-induced bone marrow aplasia. In vitro clonal assays for hematopoietic progenitor cells have been developed to assess the degree of myelotoxicity. This adverse side effect is often the limiting factor in the development of new cancer chemotherapeutics. In addition, many environmental chemicals are cytotoxic to rapidly proliferating cells, but a systematic assessment of their myelotoxicity has not been performed. We have used clonal marrow assays to investigate a panel of chemicals including 2,3,7,8-tetrachlorodibenzo-p-dioxin, polybrominated biphenyls, diethylstilbestrol, benzo(a)pyrene and indomethacin. All were immunotoxic, some to pleuripotent hemopoetic stem cells and other to granulocyte-macrophage progenitors, and at concentrations below those causing other toxic manifestations. This shows that these bone marrow clonal assays, and hopefully future one for erythroid, B- and T-lymphocytes, and megakaryocytes, will provide the specificity and sensitivity necessary to delineate the myelotoxicity of a broad spectrum of environmental chemicals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher P. W. Diethylstilbestrol metabolism in food-producing animals. J Toxicol Environ Health Suppl. 1976;1:45–59. [PubMed] [Google Scholar]
- BECKER A. J., McCULLOCH E. A., TILL J. E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963 Feb 2;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- Boggs D. R., Boggs S. S., Chervenick P. A., Patrene K. D. Murine recovery from busulfan-induced hematopoietic toxicity as assessed by three assays for colony-forming cells. Am J Hematol. 1980;8(1):43–54. doi: 10.1002/ajh.2830080106. [DOI] [PubMed] [Google Scholar]
- Boorman G. A., Luster M. I., Dean J. H., Wilson R. E. The effect of adult exposure to diethylstilbestrol in the mouse on macrophage function and numbers. J Reticuloendothel Soc. 1980 Dec;28(6):547–560. [PubMed] [Google Scholar]
- Botnick L. E., Hannon E. C., Hellman S. Limited proliferation of stem cells surviving alkylating agents. Nature. 1976 Jul 1;262(5563):68–70. doi: 10.1038/262068a0. [DOI] [PubMed] [Google Scholar]
- Botnick L. E., Hannon E. C., Hellman S. Multisystem stem cell failure after apparent recovery from alkylating agents. Cancer Res. 1978 Jul;38(7):1942–1947. [PubMed] [Google Scholar]
- Botnick L. E., Hannon E. C., Hellman S. Nature of the hemopoietic stem cell compartment and its proliferative potential. Blood Cells. 1979 Jun 15;5(2):195–210. [PubMed] [Google Scholar]
- Braunschweiger P. G., Schenken L. L., Schiffer L. M. Adriamycin-induced delayed erythropoietic injury expressed following anemia stress. Cancer Res. 1980 Jul;40(7):2257–2262. [PubMed] [Google Scholar]
- Brown C. H., 3rd, Carbone P. P. Effects of chemotherapeutic agents on normal mouse bone marrow grown in vitro. Cancer Res. 1971 Feb;31(2):185–190. [PubMed] [Google Scholar]
- Claësson M. H., Flad H. D., Opitz H. G. B and T lymphocyte colony formation in agar: 2-mercaptoethanol-activated albumin can substitute for 2-mercaptoethanol. Cell Immunol. 1979 Sep 1;46(2):398–404. doi: 10.1016/0008-8749(79)90426-x. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Golde D. W. Controlling the production of blood cells. Blood. 1979 Jan;53(1):157–165. [PubMed] [Google Scholar]
- Dean J. H., Luster M. I., Boorman G. A., Luebke R. W., Lauer L. D. The effect of adult exposure to diethylstilbestrol in the mouse: alterations in tumor susceptibility and host resistance parameters. J Reticuloendothel Soc. 1980 Dec;28(6):571–583. [PubMed] [Google Scholar]
- Delmonte L. Effect of myleran on murine hemopoiesis. I. Granulocytic cell line specificity of action on progenitor cells. Cell Tissue Kinet. 1978 Jul;11(4):347–358. [PubMed] [Google Scholar]
- Druart F., Frocrain C., Metois P., Martin J., Matuchansky C. Association of cimetidine and bone-marrow suppression in man. Dig Dis Sci. 1979 Sep;24(9):730–730. doi: 10.1007/BF01314472. [DOI] [PubMed] [Google Scholar]
- Duménil D., Sainteny F., Frindel E. Some effects of chemotherapeutic drugs on bone marrow stem cells. I. The long-term effects of phase-specific drugs on mouse bone marrow stem cells. Cancer Chemother Pharmacol. 1979;2(3):197–201. doi: 10.1007/BF00258295. [DOI] [PubMed] [Google Scholar]
- Fried W., Barone J. Residual marrow damage following therapy with cyclophosphamide. Exp Hematol. 1980 May;8(5):610–614. [PubMed] [Google Scholar]
- Gregory C. J. Erythropoietin sensitivity as a differentiation marker in the hemopoietic system: studies of three erythropoietic colony responses in culture. J Cell Physiol. 1976 Oct;89(2):289–301. doi: 10.1002/jcp.1040890212. [DOI] [PubMed] [Google Scholar]
- Haas R. J., Rohruber W., Netzel B., Lau E. Effects of CCNU on hematopoiesis in rats. Cancer Treat Rep. 1979 Mar;63(3):377–383. [PubMed] [Google Scholar]
- Hara H., Ogawa M. Erythropoietic precursors in mice under erythropoietic stimulation and suppression. Exp Hematol. 1977 Mar;5(2):141–148. [PubMed] [Google Scholar]
- Hartmann O., Parmentier C., Lemerle J., Gout M., Schweisguth O. Sequential study of the bone marrow granulocytic progenitor cells (CFC *) in children treated by chemotherapy for non-hodgkin malignant lymphomas. Nouv Rev Fr Hematol. 1979;21(3):239–250. [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R., Martin R. F., Sumner M., Fry P. Recovery of proliferating haemopoietic progenitor cells after killing by hydroxyurea. Cell Tissue Kinet. 1975 Jan;8(1):51–60. doi: 10.1111/j.1365-2184.1975.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Hoffman R., Zanjani E. D., Lutton J. D., Zalusky R., Wasserman L. R. Suppression of erythroid-colony formation by lymphocytes from patients with aplastic anemia. N Engl J Med. 1977 Jan 6;296(1):10–13. doi: 10.1056/NEJM197701062960103. [DOI] [PubMed] [Google Scholar]
- Irons R. D., Heck H., Moore B. J., Muirhead K. A. Effects of short-term benzene administration on bone marrow cell cycle kinetics in the rat. Toxicol Appl Pharmacol. 1979 Dec;51(3):399–409. doi: 10.1016/0041-008x(79)90364-8. [DOI] [PubMed] [Google Scholar]
- Jenkins V. K., Costanzi J. J., Ellis H. N. Effects of single and combined chemotherapeutic agents on hemopoietic stem cells in mice. Am J Hematol. 1976;1(1):79–88. doi: 10.1002/ajh.2830010109. [DOI] [PubMed] [Google Scholar]
- Johnston R. V., Pinkerton M. N., Mensik D. C., Swaim L. D., Linscombe V. A., Benge M. C., Barna-Lloyd G., Kilian D. J. Hematologic and myelogenous effects of inhaled benzene in the pig and the rat. J Toxicol Environ Health. 1979 Nov;5(6):1025–1035. doi: 10.1080/15287397909529811. [DOI] [PubMed] [Google Scholar]
- Levin J., Levin F. C., Metcalf D. The effects of acute thrombocytopenia on megakaryocyte-CFC and granulocyte-macrophage-CFC in mice: studies of bone marrow and spleen. Blood. 1980 Aug;56(2):274–283. [PubMed] [Google Scholar]
- Luster M. I., Boorman G. A., Dean J. H., Harris M. W., Luebke R. W., Padarathsingh M. L., Moore J. A. Examination of bone marrow, immunologic parameters and host susceptibility following pre- and postnatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Int J Immunopharmacol. 1980;2(4):301–310. doi: 10.1016/0192-0561(80)90030-2. [DOI] [PubMed] [Google Scholar]
- Maurer H. R., Henry R. Drug evaluation on haemopoietic cells in vitro. I. A micro agar colony assay with semi-automatic optical monitoring. Arzneimittelforschung. 1978;28(4):601–605. [PubMed] [Google Scholar]
- McConnell E. E., Moore J. A. Toxicopathology characteristics of the halogenated aromatics. Ann N Y Acad Sci. 1979 May 31;320:138–150. [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Metcalf D., Nossal G. J., Warner N. L., Miller J. F., Mandel T. E., Layton J. E., Gutman G. A. Growth of B-lymphocyte colonies in vitro. J Exp Med. 1975 Dec 1;142(6):1534–1549. doi: 10.1084/jem.142.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., Gross M. A., Yunis A. A. Heterogeneity of human colony-forming cells (CFUc) with respect to their sensitivity to chloramphenicol. Exp Hematol. 1980 Mar;8(3):236–242. [PubMed] [Google Scholar]
- Moore M. A., Williams N., Metcalf D. Purification and characterisation of the in vitro colony forming cell in monkey hemopoietic tissue. J Cell Physiol. 1972 Apr;79(2):283–292. doi: 10.1002/jcp.1040790213. [DOI] [PubMed] [Google Scholar]
- Morley A., Blake J. An animal model of chronic aplastic marrow failure. I. Late marrow failure after busulfan. Blood. 1974 Jul;44(1):49–56. [PubMed] [Google Scholar]
- Nathan D. G., Hillman D. G., Chess L., Alter B. P., Clarke B. J., Breard J., Housman D. E. Normal erythropoietic helper T cells in congenital hypoplastic (Diamond-Blackfan) anemia. N Engl J Med. 1978 May 11;298(19):1049–1051. doi: 10.1056/NEJM197805112981903. [DOI] [PubMed] [Google Scholar]
- Pannacciulli I. M., Massa G. G., Saviane A. G., Ghio R. L., Bianchi G. L., Bogliolo G. V. Effect of bleeding on in vivo in vitro colony-forming hemopoietic cells. Acta Haematol. 1977;58(1):27–33. doi: 10.1159/000207802. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Quesenberry P., Levitt L. Hematopoietic stem cells (third of three parts). N Engl J Med. 1979 Oct 18;301(16):868–872. doi: 10.1056/NEJM197910183011605. [DOI] [PubMed] [Google Scholar]
- Snyder C. A., Goldstein B. D., Sellakumar A. R., Bromberg I., Laskin S., Albert R. E. The inhalation toxicology of benzene: incidence of hematopoietic neoplasms and hematotoxicity in ARK/J and C57BL/6J mice. Toxicol Appl Pharmacol. 1980 Jun 30;54(2):323–331. doi: 10.1016/0041-008x(80)90202-1. [DOI] [PubMed] [Google Scholar]
- Spivak J. L., Graber S. Erythropoietin and the regulation of erythropoiesis. Johns Hopkins Med J. 1980 Jun;146(6):311–320. [PubMed] [Google Scholar]
- Strom S., Johnson R. L., Uyeki E. M. Mercury toxicity to hemopoietic and tumor colony-forming cells and its reversal by selenium in vitro. Toxicol Appl Pharmacol. 1979 Jul;49(3):431–436. doi: 10.1016/0041-008x(79)90443-5. [DOI] [PubMed] [Google Scholar]
- Strom S., Johnson R. L., Uyeki E. M. Mercury toxicity to hemopoietic and tumor colony-forming cells and its reversal by selenium in vitro. Toxicol Appl Pharmacol. 1979 Jul;49(3):431–436. doi: 10.1016/0041-008x(79)90443-5. [DOI] [PubMed] [Google Scholar]
- Synder C. A., Goldstein B. D., Sellakumar A., Wolman S. R., Bromberg I., Erlichman M. N., Laskin S. Hematotoxicity of inhaled benzene to Sprague-Dawley rats and AKR mice at 300 ppm. J Toxicol Environ Health. 1978 Jul;4(4):605–618. doi: 10.1080/15287397809529683. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Udupa K. B., Reissmann K. R. Cell kinetics of erythroid colony-forming cells (CFU-E) studied by hydroxyurea injections and sedimentation velocity profile. Exp Hematol. 1978 Apr;6(4):398–404. [PubMed] [Google Scholar]
- Vos O., Dolmans M. J. Self-renewal of colony forming units (CFU) in serial bone marrow transplantation experiments. Cell Tissue Kinet. 1972 Sep;5(5):371–385. doi: 10.1111/j.1365-2184.1972.tb00376.x. [DOI] [PubMed] [Google Scholar]


