Abstract
Poor fetal environments are thought to produce adaptive changes in human developmental trajectories according to the Predictive Adaptive Response hypothesis. Although many studies have demonstrated correlations between indicators of fetal environment and negative adult health outcomes, the adaptive significance of these outcomes is unclear. Our study explicitly tests the adaptive nature of fetal programming in humans. We show that differences in nutritional status at birth are associated with adaptive differences in the sensitivity of adult ovarian function to energetic stress. Women who were born as relatively fat babies do not exhibit ovarian suppression in response to moderate levels of physical activity at adulthood, in contrast to women who were born as skinnier babies. The levels of estradiol in women born in the highest tertile of ponderal index (an indicator of neonatal nutritional status) were 37% and 46% higher, respectively, than levels of estradiol in women born in the low and middle ponderal index tertiles. These findings suggest that fetal programming of reproductive function results in developmentally plastic, but essentially adaptive, shifts in set points of ovarian response to energetic stress, such that women who were gestated under conditions of energetic constraint show greater sensitivity to energetic stress in adulthood. Our results have practical implications in terms of behavioral strategies for reducing the risk of breast cancer. We suggest that the amount of activity necessary to reduce levels of estrogen, which may in turn reduce cancer risk, can depend on a woman's nutritional status at birth.
Keywords: birth weight, cancer prevention, fetal programming, ovarian function, physical activity
Human biologists have long recognized developmental plasticity as an important adaptive characteristic of human growth and development (1). Recently, epidemiological studies have demonstrated particularly strong relationships between indices of fetal development, such as size at birth, and health outcomes associated with chronic disease conditions decades later (2–5). These observations have led to the advance of the Predictive Adaptive Response (PAR) hypothesis, which posits that conditions experienced by the human embryo and fetus affect subsequent trajectories of development and set points for physiological function in various systems in ways that would promote the fitness of the developing organism in later years (6–8). In particular, it is hypothesized that conditions of energy constraint during gestation result in physiological changes that improve the fitness of the adult organism in energy-restricted environments. The adaptive nature of these changes is difficult to demonstrate, however, because the outcomes associated with poor fetal environments are most often associated with increased incidence and severity of pathologies that compromise life expectancy, such as hypertension or type II diabetes. A second hypothesis, the Environmental Mismatch (EM) hypothesis, must be posited, supplementing the PAR hypothesis, to explain why a process that is supposed to be adaptive results in outcomes that are maladaptive (8). The EM hypothesis suggests that these poor outcomes result from the fact that fetal environments, although formerly having a predictive, positive correlation with childhood and adult environmental conditions in the formative past of human evolution, either no longer retain this predictive quality or do not retain it to the same degree. Thus, it is argued, the physiological changes that result from fetal programming often produce pathology rather than adaptation in the modern context.
Although the logic of the PAR and EM hypotheses is attractive, it is difficult to think of how they can be tested in combination. By itself, the PAR would be falsified if the results of fetal programming could be shown to be maladaptive. The EM hypothesis, however, explains away such falsification, leaving open the question of how to render the joint PAR/EM hypothesis falsifiable. It is particularly difficult to see how the predictions of the joint PAR/EM hypothesis can be distinguished from major competing hypotheses. The most obvious competing hypothesis is that poor or constrained fetal environments produce congenital pathologies or disruptions of normal developmental processes, resulting in pathological outcomes. Thus, both the joint PAR/EM hypothesis and its major alternative make the same predictions.
We present a different logical approach to a test of the PAR hypothesis in the well developed theoretical context of human reproductive ecology (9–15). Reproductive ecology combines the study of human reproductive physiology with the theoretical structure of life history theory (16). From this perspective, reproductive function in humans is viewed as facultatively responsive to different ecological circumstances in ways that increase individual fitness. Rather than having only one optimal state, reproductive function is considered to reflect a norm of reaction to varying environmental conditions. Among the most important environmental factors affecting ovarian function in women are factors affecting metabolic energy availability (17). When energy availability is constrained, either by limited intake or by increased expenditure (e.g., exercise or physical labor), indices of ovarian function associated with the probability of conception, such as ovarian steroid hormone levels, length of menstrual cycles, and cycle regularity, shift toward lower conception probability. When energy availability is high, as evidenced by weight or fat gain or increasing insulin levels, indices of ovarian function shift toward high conception probability. This pattern of response is considered adaptive, because it modulates conception probability in response to conditions that favor successful pregnancy outcomes (18). This norm of reaction of ovarian function to energetic conditions has been demonstrated across a broad range of human populations, as well as a broad range of genetic, environmental, and cultural contexts, and as such appears to be a general, evolved feature of human biology (9, 17).
Assays of salivary steroid levels have proven to be particularly sensitive in documenting shifts in ovarian function in response to energy availability (19). Because steroid levels in saliva represent free, rather than free-plus-bound circulating steroid levels, they allow for much finer discrimination of functional differences in steroid signaling. Because saliva can be readily collected from subjects on multiple occasions, it is possible to compare steroid levels across entire menstrual cycles among different women rather than relying on one or a few timed blood samples. Salivary levels of ovarian steroids have been used by many investigators to demonstrate adaptive shifts in ovarian function under conditions of energy constraint in adult women (15, 19–27). Salivary estradiol (E2) levels are particularly sensitive indicators, with a shift to lower levels being associated with energy constraint and lower probability of conception (18).
The framework of reproductive ecology provides for a clear test of the PAR hypothesis without having to evoke EM to account for pathological outcomes. Within that framework, we expect that constrained energy availability will result in a shift to lower conception probability, reflected in a shift to lower levels of salivary E2. The direction of this predicted shift has been established by previous studies. Therefore, if natural selection has designed fetal physiology to interpret constrained energy availability in utero as predictive of constrained energy availability ex utero, we predict that the norm of reaction for adult ovarian function with respect to energy availability should be shifted toward greater sensitivity to low energy availability. That is, we predict that women whose own fetal development was constrained by energy availability should be more sensitive to limited energy availability as adults than women whose own fetal development was not constrained. This prediction can be clearly distinguished from the results of pathological disruption of ovarian function, which would result in either general reproductive failure or loss of a functional responsiveness to environmental energy availability.
Using data from 106 Polish women, each monitored for a full menstrual cycle, we show that nutritional status at birth is a predictor of the sensitivity of adult ovarian function to energetic stress. Women who had a high ponderal index (PI, an indicator of nutritional status, calculated as body weight/body length3) at birth do not exhibit ovarian suppression in response to moderate levels of physical activity (PA) at adulthood but do in response to higher levels of PA, whereas women who had a lower PI at birth show ovarian suppression in association with moderate energetic stress.
Results
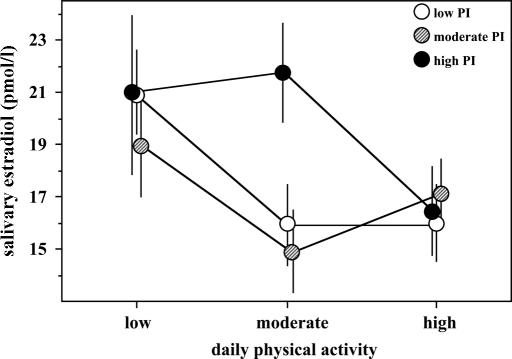
At low PA, all PI tertiles had similar levels of E2; similarly the PI tertiles did not differ at high PA (Fig. 1). At moderate PA, mean E2 levels were significantly higher in women from the third PI tertile than in those from the first (F = 22.33, P = 0.0001) and second (F = 25.20, P = 0.0001) tertiles. Women fat at birth (third tertile) had unsuppressed levels of E2 at the moderate PA level, compared with low PA (F = 0.32, P = 0.571), whereas women skinnier at birth had significantly reduced E2 levels (the difference between low PA and moderate PA for the first PI tertile: F = 16.2, P = 0.0001; for the second PI tertile, the difference is marginally significant: F = 7.00, P = 0.008). Whereas there were no effects of increased activity level from moderate PA to high PA for the two lower PI tertiles (first: F = 0.022, P = 0.881; second: F = 2.47, P = 0.116), there was a significant and pronounced reduction in mean E2 levels for women fat at birth (third tertile; F = 8.757, P = 0.003). PI did not show any statistically significant relationship with nutritional status at adulthood (correlation with body weight r = 0.11, P = 0.27; with body mass index r = 0.1, P = 0.31, and with percentage of body fat r = 0.09, P = 0.35).
Fig. 1.
Mean (with 95% confidence intervals) levels of E2 in women from PI tertiles in three different levels of mean daily PA.
Baseline characteristics of the study subjects are presented in Table 1. The three groups characterized by different PI did not show statistically significant variation in factors that can potentially influence levels of hormones in menstrual cycles, such as age, age at first menstruation, nutritional status at adulthood, and PA.
Table 1.
Mean (±SD) characteristics of study subjects divided into low, moderate, and high tertiles according to their PI at birth
| Characteristic | Low PI* | Moderate PI† | High PI‡ | P§ |
|---|---|---|---|---|
| Age, years | 29.2 (3.95) | 29.0 (3.55) | 30.1 (3.04) | 0.37 |
| Self-reported menarcheal age, years | 13.6 (1.18) | 13.2 (1.17) | 13.8 (1.33) | 0.30 |
| Body height, cm | 164.0 (6.98) | 161.8 (5.73) | 162.2 (6.3) | 0.30 |
| Body weight, kg | 63.1 (11.82) | 60.7 (10.41) | 59.7 (7.35) | 0.34 |
| Body mass index, kg/m2 | 23.4 (3.97) | 23.2 (3.77) | 22.7 (2.62) | 0.69 |
| Body fat, % | 28.1 (7.87) | 26.7 (7.3) | 26.4 (6.19) | 0.54 |
| Length of menstrual cycle, days | 28.6 (3.87) | 27.7 (3.03) | 29.6 (3.67) | 0.08 |
| Physical activity, MET-h/day | 44.7 (15.11) | 44.1 (11.32) | 40.9 (10.45) | 0.38 |
MET, metabolic equivalent.
*Mean = 18.08 kg/m3; n = 35.
†Mean = 21.15 kg/m3; n = 35.
‡Mean = 24.79 kg/m3; n = 36.
§P values denote significance of variation among the three groups (from ANOVA).
Discussion
The central claim of the PAR hypothesis is that developmental changes that are associated with energy constraints on fetal growth are essentially adaptive, preparing the organism for a predicted range of environments it is likely to encounter in adult life. A host of studies have demonstrated that energetic constraints on fetal development in humans and other mammals are indeed associated with altered physiology later in life (28–30). However, because most of the outcomes that are investigated in relation to fetal growth retardation are expressed as increased incidence and severity of pathologies later in life, the claim that they are adaptive is problematic. In particular, it is difficult to test the claim of adaptive change when a separate hypothesis, the EM hypothesis, is often evoked to account for maladaptive outcomes.
We used the framework of reproductive ecology to generate a specific prediction about changes in the sensitivity of adult ovarian function to energetic stress in response to energetic constraints on fetal development. We predicted that, if the PAR hypothesis holds, ovarian sensitivity to energetic constraints should be heightened in women whose own fetal growth was energetically constrained. If the major alternative hypothesis, that the effects of energetic constraints on fetal development are pathological, were to hold, however, we would predict general disruption of ovarian function or a disruption of ovarian responsiveness to energetic constraint in adulthood. An adaptive shift in the norm of reaction of ovarian E2 levels to energy availability preserves ovarian function and responsiveness to energetic conditions, enhancing fitness in the energy-constrained environment that an energy-constrained fetus expects. Pathological disruption of ovarian function, however, sacrifices fecundity without regard to environmental circumstances, to the detriment of fitness.
This study represents, to our knowledge, the first direct test of the adaptive nature of fetal programming in humans. Previous studies have documented deleterious effects of fetal growth retardation on various aspects of reproductive physiology in humans. In human males, small size at birth correlates with reduced testicular volume, lower testosterone and luteinizing hormone levels (31), and also low adult fertility (32). In human females, reduced fetal growth has been associated with impaired ovarian development (33), reduced uterine and ovarian size, and anovulation in adolescent girls (34, 35). These outcomes appear to be pathological, however, and deleterious to reproductive fitness. We have previously shown that women with a high PI at birth have 22% higher levels of E2 in menstrual cycles than women with a lower PI (36). But the adaptive significance of this relationship between PI and adult E2 levels is not clear by itself. It might be that low PI is simply associated with generally constrained ovarian function rather than a shift in the norm of reaction to energetic conditions. The adaptive nature of this change in adult ovarian function becomes apparent when the norm of reaction of adult ovarian function to energetic constraint is considered rather than average function or function in a single energetic context.
A variety of indices have been used in different studies to indicate energetic constraint on fetal development (37–40). PI was used by Barker (3) and others in early studies of fetal growth and cardiovascular disease (41), blood pressure (42), insulin resistance (43), glucose tolerance (44), and maternal diet in pregnancy (45). PI, in our view, is preferable to birth weight as an index of energetic constraint on fetal development for our study because it reflects moderate degrees of energetic challenge that constrain fetal fat accumulation late in pregnancy rather than more severe limitations that constrain skeletal and organ development earlier in pregnancy. Ovarian function has been shown to respond quantitatively to moderate levels of energetic stress, whereas extreme energetic stress can result in complete ovarian quiescence. Thus, environments that result in low PI should correspond to environments that result in down-regulated ovarian function.
In addition to providing an explicit test of the PAR hypothesis, these results carry important practical implications in the domain of reproductive health. Ovarian steroid hormones are implicated in the development and growth of breast cancer (46–48). Size at birth, by influencing adult hormonal levels (36), may impact the adult risk of breast cancer: women with small size at birth have reduced risk (49). Engaging in regular PA has been proposed as an important strategy capable of reducing the risk of breast cancer (50–52). Our results suggest that the recommended intensity and duration of PA necessary to lower endogenous levels of estrogens and consequently to reduce the risk of breast cancer may depend on woman's nutritional status at birth. More specifically, although moderate PA levels are associated with reduced levels of E2 in women born with lower PI, such levels of activity may not be sufficient to suppress ovarian function in women born with higher PI. Therefore, in such women, higher PA levels may be necessary for a reduction in the risk of breast cancer.
Methods
Study Group.
We tested the relationship between PA and daily salivary E2 levels across complete menstrual cycles in women who differed in PI at birth: 106 Polish women, average age 29.5 years, participated in the study (Table 1). Women were recruited for the study by advertisements and selected for participation if they met the following criteria: between 24 and 36 years old, regular menstrual cycles and no fertility problems, no gynecological and/or chronic disorders (i.e., diabetes, hypo/hyperthyroidism), not taking any hormonal medication or using hormonal contraception during the 6 months before recruitment, and not pregnant or lactating during the 6 months before recruitment. Recruited women signed a consent form after being informed of the aims and requirements of the study, which had been approved by the Jagiellonian University Research Ethics Committee.
Anthropometric Measurements, General Questionnaire, and Birth Characteristics.
Subjects' body weight, height, subscapular and triceps skinfolds, and percent body fat (by bioimpedance) were measured by a trained anthropologist. A general questionnaire (part interview and part self-administered) was used to collect information on education, reproductive history, family history, and past use of hormonal medication. Data on birth weight and birth length were obtained from subjects' personal health books, which contain the individual's birth size, health condition, and any parturition problems (recorded at birth).
E2 Assay Procedure.
Women collected daily morning saliva samples for one entire menstrual cycle. Saliva samples from 20 days (reverse cycle days −5 to −24, where the last day of each cycle was marked as day −1) of each cycle were analyzed for the concentration of E2 by using an 125I-based RIA kit (no. 39100; Diagnostic Systems Laboratories, Webster, TX) with published modifications (53) to the manufacturer's protocol. The sensitivity of the E2 assay is 4 pmol/liter. Average intraassay variability was 9%, and interassay variability ranged from 23% for lower (15 pmol/liter) to 13% for higher (50 pmol/liter) values.
PA.
PA was assessed based on a preset daily log of PA that was completed by the subjects every day during the menstrual cycle. Women recorded number of hours of sleep, time of waking, and number of minutes spent each day in five categories of PA: walking to and from work; other walking; physical work at home; physical occupational work; and exercise and sports participation. Women were asked to assign the intensity level (from 1 to 4) for each of the listed activities according to provided definitions (based on changes in pulse and sweating). The period of inactivity was assessed by adding the number of hours of sleep and time performing activities of intensity 1 to intensity 4, and subtracting the sum from 24 h (54). The category of inactivity included all activities that subjects did not list as PA, for example, most sedentary activities at home and at work, watching television, and reading. For each class of activities the following metabolic equivalents (METs) were assigned: sleep = 1 MET; inactivity = 1.1 METs; activities of intensity 1 = 1.5 METs; activities of intensity 2 = 4 METs; activities of intensity 3 = 6 METs; and activities of intensity 4 = 10 METs (54, 55). Mean 24-h PA (in MET-h/day) was calculated for each subject.
Variation in the mean PA levels among tertiles resulted from variation in time spent in housework and occupational work (23). All pairwise differences among groups are statistically significant for these two categories of PA. Groups with low, moderate, and high PA did not differ in mean time of walking to and from work and in mean time of other walking. The three groups differed marginally in mean time performing exercise-related activities. The moderate-activity group spent more time in sport/exercise than the low-activity group (P = 0.01), but all other differences in sport/exercise among groups were not statistically significant (23).
Statistical Analysis.
Women were divided into tertiles of PA based on their mean levels of habitual, daily PA recorded during one entire menstrual cycle (23). The women were also divided into tertiles of PI at birth based on individual medical records of weight and length at birth (36). Mean levels of E2 were compared in pairwise contrasts (each at P = 0.003, with Bonferroni correction) after a three-way fixed-model ANOVA, with PI tertiles (3 levels), PA tertiles (3 levels), and days of menstrual cycle (18 levels) as three factors. Interactions with cycle-days were all nonsignificant (P > 0.7) and were removed from the model.
Acknowledgments
This work was supported by the State Committee for Scientific Research, Poland, and by the Radcliffe Institute for Advanced Study at Harvard University (G.J.).
Abbreviations
- EM
environmental mismatch
- E2
estradiol
- MET
metabolic equivalent
- PA
physical activity
- PAR
Predictive Adaptive Response
- PI
ponderal index.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Lasker G. W. Science. 1969;166:1480. doi: 10.1126/science.166.3912.1480. [DOI] [PubMed] [Google Scholar]
- 2.Barker D. J. P., Osmond C., Golding J., Kuh D., Wadsworth M. E. J. Br. Med. J. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker D. Mothers, Babies, and Disease in Later Life. London: BMJ; 1994. [Google Scholar]
- 4.Kuzawa C. W., Adair L. S. Am. J. Clin. Nutr. 2003;77:960–966. doi: 10.1093/ajcn/77.4.960. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson M., Wallander M. A., Krakau I., Wedel H., Svardsudd K. J. Intern. Med. 2004;255:236–246. doi: 10.1046/j.1365-2796.2003.01289.x. [DOI] [PubMed] [Google Scholar]
- 6.Mouseau T., Fox C. Maternal Effects as Adaptations. New York: Oxford Univ. Press; 1998. [Google Scholar]
- 7.Bateson P., Barker D., Clutton-Brock T., Deb D., D'Udine B., Foley R. A., Gluckman P., Godfrey K., Kirkwood T., Lahr M. M., et al. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman P. D., Hanson M. A. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 9.Ellison P. T., Panter B. C., Lipson S. F., O'Rourke M. T. Hum. Reprod. 1993;8:2248–2258. doi: 10.1093/oxfordjournals.humrep.a138015. [DOI] [PubMed] [Google Scholar]
- 10.Ellison P. T. Annu. Rev. Anthropol. 1994;23:255–275. doi: 10.1146/annurev.an.23.100194.001351. [DOI] [PubMed] [Google Scholar]
- 11.Ellison P. T. Am. J. Hum. Biol. 2003;15:342–351. doi: 10.1002/ajhb.10152. [DOI] [PubMed] [Google Scholar]
- 12.Jasienska G. Acta Biotheor. 2003;51:1–18. doi: 10.1023/a:1023035321162. [DOI] [PubMed] [Google Scholar]
- 13.Jasienska G. In: Reproductive Ecology and Human Evolution. Ellison P. T., editor. Hawthorne, NY: Aldine de Gruyter; 2001. pp. 59–85. [Google Scholar]
- 14.Jasienska G., Ellison P. T. Am. J. Hum. Biol. 2004;16:563–580. doi: 10.1002/ajhb.20063. [DOI] [PubMed] [Google Scholar]
- 15.Jasienska G., Ellison P. T. Proc. R. Soc. London Ser. B; 1998. pp. 1847–1851. [Google Scholar]
- 16.Ellison P. T., editor. Reproductive Ecology and Human Evolution. Hawthorne, NY: Aldine De Gruyter; 2001. [Google Scholar]
- 17.Ellison P. T. On Fertile Ground. Cambridge, MA: Harvard Univ. Press; 2003. [Google Scholar]
- 18.Lipson S. F., Ellison P. T. Hum. Reprod. 1996;11:2090–2096. doi: 10.1093/oxfordjournals.humrep.a019055. [DOI] [PubMed] [Google Scholar]
- 19.Ellison P. T. Ann. N.Y. Acad. Sci. 1994;709:287–298. doi: 10.1111/j.1749-6632.1994.tb30417.x. [DOI] [PubMed] [Google Scholar]
- 20.Ellison P. T., Lager C. Am. J. Obstet. Gynecol. 1986;154:1000–1003. doi: 10.1016/0002-9378(86)90737-4. [DOI] [PubMed] [Google Scholar]
- 21.Ellison P. T., Peacock N. R., Lager C. Am. J. Phys. Anthropol. 1989;78:519–526. doi: 10.1002/ajpa.1330780407. [DOI] [PubMed] [Google Scholar]
- 22.Panter-Brick C., Lotstein D. S., Ellison P. T. Hum. Reprod. 1993;8:684–690. doi: 10.1093/oxfordjournals.humrep.a138120. [DOI] [PubMed] [Google Scholar]
- 23.Jasienska G., Ziomkiewicz A., Thune I., Lipson S. F., Ellison P. T. Eur. J. Cancer Prev. 2006 doi: 10.1097/00008469-200610000-00009. in press. [DOI] [PubMed] [Google Scholar]
- 24.Rosetta L., Harrison G. A., Read G. F. Ann. Hum. Biol. 1998;25:345–357. doi: 10.1080/03014469800005692. [DOI] [PubMed] [Google Scholar]
- 25.Lebenstedt M., Platte P., Pirke K. M. Med. Sci. Sports Exerc. 1999;31:1250–1256. doi: 10.1097/00005768-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Vitzthum V. J., Bentley G. R., Spielvogel H., Caceres E., Thornburg J., Jones L., Shore S., Hodges K. R., Chatterton R. T. Hum. Reprod. 2002;17:1906–1913. doi: 10.1093/humrep/17.7.1906. [DOI] [PubMed] [Google Scholar]
- 27.Reading K. J., McCargar L. J., Harber V. J. Int. J. Sport Nutr. Exerc. Metab. 2002;12:93–104. doi: 10.1123/ijsnem.12.1.93. [DOI] [PubMed] [Google Scholar]
- 28.Gluckman P. D., Hanson M. A. Trends Endocrinol. Metab. 2004;15:183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Gluckman P. D., Hanson M. A. The Fetal Matrix: Evolution, Development and Disease. Cambridge, U.K.: Cambridge Univ. Press; 2005. [Google Scholar]
- 30.Kuzawa C. W. Am. J. Hum. Biol. 2005;17:5–21. doi: 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- 31.Cicognani A., Alessandroni R., Pasini A., Pirazzoli P., Cassio A., Barbieri E., Cacciari E. J. Pediatr. 2002;141:376–380. doi: 10.1067/mpd.2002.126300. [DOI] [PubMed] [Google Scholar]
- 32.Francois I., deZegher F., Spiessens C., Dhooghe T., Vanderschueren D. Pediatr. Res. 1997;42:899–901. doi: 10.1203/00006450-199712000-00029. [DOI] [PubMed] [Google Scholar]
- 33.de Bruin J. P., Dorland M., Bruinse H. W., Spliet W., Nikkels P. G. J., Te Velde E. R. Early Hum. Dev. 1998;51:39–46. doi: 10.1016/s0378-3782(97)00073-x. [DOI] [PubMed] [Google Scholar]
- 34.Ibanez L., Potau N., Enriquez G., De Zegher F. Pediatr. Res. 2000;47:575–577. doi: 10.1203/00006450-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Ibanez L., Potau N., Ferrer A., Rodriguez-Hierro F., Marcos M. V., De Zegher F. J. Clin. Endocrinol. Metab. 2002;87:3391–3393. doi: 10.1210/jcem.87.7.8657. [DOI] [PubMed] [Google Scholar]
- 36.Jasienska G., Ziomkiewcz A., Lipson S. F., Thune I., Ellison P. T. Am. J. Hum. Biol. 2006;18:133–140. doi: 10.1002/ajhb.20462. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein I. Curr. Opin. Clin. Nutr. Metab. 2005;8:613–617. doi: 10.1097/01.mco.0000170757.78737.74. [DOI] [PubMed] [Google Scholar]
- 38.Tamim H., Beydoun H., Itani M., Khogali M., Chokr I., Yunis K. A. J. Perinat. Med. 2004;32:509–513. doi: 10.1515/JPM.2004.120. [DOI] [PubMed] [Google Scholar]
- 39.Dombrowski M. P., Berry S. M., Johnson M. P., Saleh A. A. A., Sokol R. J. Arch. Pediatr. Adolesc. Med. 1994;148:508–512. doi: 10.1001/archpedi.1994.02170050066012. [DOI] [PubMed] [Google Scholar]
- 40.Walther F. J., Ramaekers L. H. J. J. Perinat. Med. 1982;10:42–47. doi: 10.1515/jpme.1982.10.1.42. [DOI] [PubMed] [Google Scholar]
- 41.Barker D. J. P., Osmond C., Simmonds S. J., Wield G. A. Br. Med. J. 1993;306:422–426. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorensen H. T., Thulstrup A. M., Norgard B., Engberg M., Madsen K. M., Johnsen S. P., Olsen J., Lauritzen T. Scand. Cardiovasc. J. 2000;34:390–395. doi: 10.1080/14017430050196216. [DOI] [PubMed] [Google Scholar]
- 43.Phillips D. I. W., Barker D. J. P., Hales C. N., Hirst S., Osmond C. Diabetologia. 1994;37:150–154. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 44.Law C. M., Gordon G. S., Shiell A. W., Barker D. J. P., Hales C. N. Diabet. Med. 1995;12:24–29. doi: 10.1111/j.1464-5491.1995.tb02057.x. [DOI] [PubMed] [Google Scholar]
- 45.Godfrey K. M., Barker D. J. P., Robinson S., Osmond C. Br. J. Obstet. Gynaecol. 1997;104:663–667. doi: 10.1111/j.1471-0528.1997.tb11975.x. [DOI] [PubMed] [Google Scholar]
- 46.Pike M. C., Spicer D. V., Dahmoush L., Press M. F. Epidemiol. Rev. 1993;15:17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 47.Jasienska G., Thune I., Ellison P. T. Eur. J. Cancer Prev. 2000;9:231–239. doi: 10.1097/00008469-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Ellison P. T. In: Hormones, Health, and Behavior. A Socio-ecological and Lifespan Perspective. Panter-Brick C., Worthman C. M., editors. Cambridge, U.K.: Cambridge Univ. Press; 1999. pp. 184–209. [Google Scholar]
- 49.Michels K. B., Trichopoulos D., Robins J. M., Rosner B. A., Manson J. E., Hunter D. J., Colditz G. A., Hankinson S. E., Speizer F. E., Willett W. C. Lancet. 1996;348:1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- 50.Bernstein L., Ross R. K., Henderson B. E. Am. J. Epidemiol. 1992;135:142–152. doi: 10.1093/oxfordjournals.aje.a116267. [DOI] [PubMed] [Google Scholar]
- 51.Friedenreich C. M., Rohan T. E. Epidemiology. 1995;6:311–317. doi: 10.1097/00001648-199505000-00021. [DOI] [PubMed] [Google Scholar]
- 52.Thune I., Brenn T., Lund E., Gaard M. N. Engl. J. Med. 1997;336:1269–1275. doi: 10.1056/NEJM199705013361801. [DOI] [PubMed] [Google Scholar]
- 53.Jasienska G., Ziomkiewicz A., Ellison P. T., Lipson S. F., Thune I. Proc. R. Soc. London Ser. B; 2004. pp. 1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson M. T., Ainsworth B. E., Jacobs J. R., Leon A. S. Ann. Epidemiol. 2001;11:145–153. doi: 10.1016/s1047-2797(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 55.Ainsworth B. E., Haskel W. L., Whitt M. C., Irwin M. L., Swartz A. M., Strath S. J., O'Brien W. L., Bassett D. R., Schmitz K. H., Emplaincourt P. O., et al. Med. Sci. Sports Exerc. 2000;32:S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]