Abstract
The purpose of the present work is to study the functional roles of two predefined regions of interest: one in the left anterior cingulate cortex (ACC) that seems to reflect goal-relevant control demand, and one in the left prefrontal cortex (PFC) that reflects memory retrieval demand. Two slow event-related brain imaging experiments were conducted, adapting a cognitive skill acquisition paradigm. Experiment 1 found that both left ACC and left PFC activity increased parametrically with task difficulty. Using a slight modification of the same basic paradigm, Experiment 2 attempted to decouple retrieval and control demands over the course of learning. Participants were imaged early in training and again several days later, after substantial additional training in the task. There was a clear dissociation between activity in the left PFC and the left ACC. Although the PFC region showed a substantial decrease in activity over the course of learning, reflecting greater ease of retrieval, the ACC showed the opposite pattern of results with significantly greater activity after training, reflecting increased control demand. Moreover, the increased response in the ACC occurred when errors and latencies were smallest.
Keywords: control, functional MRI, learning, retrieval
Studies of cognitive neuroimaging have consistently shown that the anterior cingulate cortex (ACC) and lateral areas within the prefrontal cortex (PFC) are critically active when participants are engaged in cognitively demanding tasks (1, 2). However, researchers in the field are still trying to articulate the precise roles of medial and lateral frontal areas in terms of cognitive demand and control. The present work uses event-related functional MRI (fMRI) to investigate two particular components of cognitive demand: control demand and retrieval demand. The studies reported here specify a priori two frontal cortical regions of interest (ROIs) whose responses seem to reflect these component processes: an ROI in the left ACC that we believe reflects goal-relevant control demand and an ROI in the left PFC that we believe reflects memory retrieval demand.
The first experiment shows that the activity of both the ACC and PFC regions increases similarly with overall cognitive demand. This result reflects a common feature of cognitive tasks: more difficult problems tend to increase demand across many, if not all, constituent cognitive processes. The second experiment tries to separate the function of these regions by using a learning paradigm. The predominant result in cognitive neuroimaging studies of learning is that, over the course of practice, many brain areas show decreases in activity, attributed to increased neural efficiency (3) or to reduced cognitive demand and control requirements (4). However, the second experiment shows an increase in ACC activity and a decrease in PFC activity.
Predefined ROIs.
The ACC has been the focus of a great deal of imaging work in the context of its role in cognitive control and its relationship to other frontal brain areas in the service of control (2, 5–7). A variety of theories have been proposed for the role of ACC in cognition. Some theories (8–10) have postulated that it is involved in top-down control of cognition, whereas other theories have related ACC activity to very specific functions such as error detection. Its role in error detection is supported by error-related negativity in event-related potentials observed when errors are made in speeded response tasks (11, 12). However, ACC activity occurs in many more situations than when there are errors, and another interpretation of its activity is that it is a reflection of task difficulty as indexed by errors or reaction time (13). Another view has been proposed (14, 15) that the true function of ACC is monitoring for conflict among potential responses, as occurs in the Stroop task (16–18), and that other regions of the cortex actually respond to the detected conflict. However, we have recently shown that ACC activity can reflect some sort of cognitive demands that do not involve any overt responses or competition among responses (19). Along this line, a more general view has begun to emerge suggesting ACC is responsive not simply to response conflict but also to more general types of conflict, such as semantic (20) or retrieval conflict (21).
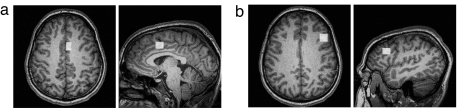
Based on this research, we have proposed that ACC serves a related but more general role than conflict detection in cognition (22): it implements the goal module in the ACT-R cognitive modeling architecture (23), the responsibility of which is to update abstract control states in tasks that require following a course of mental activity independent of the external situation. For example, while mentally solving an algebra problem (22), it is necessary to keep track of where one is in the solution of the problem. More generally, whenever a branch point in a mental sequencing of operations needs to be marked, an additional control state is required. Such conditions arise, for example, when one must wait for a retrieval operation to complete before continuing with a mental computation, or when one needs to evaluate which of a number of alternative solution paths to follow. The ROI in the current work is a predefined ACC region shown by previous studies in our laboratory to reflect this module [Brodmann's area (BA) 24/32, a 9 × 16 × 13-mm3 region centered at −6, 10, 39 in Talairach coordinates; Fig. 1a].
Fig. 1.
Axial and saggital views of predefined ROIs for ACC (Brodmann's area 24/32, centered at −6, 10, 39 in Talairach coordinates, a) and PFC (Brodmann's area 9/46, centered at −42, 23, 24 in Talairach coordinates, b). ROIs are superimposed on a T1-weighted structural image of the reference brain used in subsequent analyses. Axial views are in radiological space (left image = right brain). Note that ROIs are left-lateralized.
The PFC is a large region of the cortex, and areas within the PFC have been shown to be differentially active across a wide range of cognitive tasks (24, 25). The subregion of PFC of interest in this paper has been shown to reflect both control processes (16, 26–28) as well as memory retrieval and encoding processes (24, 25, 29–35). Consistent with these studies, we have defined a PFC ROI (a box 16 × 16 × 13 mm3 centered at −42, 23, 24 in Talairach coordinates corresponding to Brodmann's area 9/46; Fig. 1b). We have demonstrated across a variety of studies that this region increases in activity as retrieval demands increase (36–40).
Experimental Overview
The current experiments have their origins in work studying the behavioral changes that take place when one acquires a new skill, transitioning from the state of novice to expert in a complex task (41, 42). The task involved initially learning facts about pairs of times for sports events. For instance, a participant might be told that the first hockey game played was on Monday at 3:00 p.m., and the second game was played on Wednesday at 2:00 p.m. After memorizing a small set of facts like this one for different sports, participants later were told to extract from those facts rules about the timing of the two events. For instance, in this case, the second hockey game was 2 days later (Monday + 2 = Wednesday) and 1 hr earlier (3:00 p.m. − 1 = 2:00 p.m.), so hockey was a “+2, −1” rule. Each sport was uniquely associated with a single rule. Participants had to extract this type of rule for each sport and apply the rules to new cases. So, they might be asked when the second hockey game was if the first game had been played on Tuesday at 3:00 p.m. (answer: Thursday at 2:00 p.m.). For the purposes of the present research, there are two important features. The first is that there is dramatic speedup over the course of days of practice on a set of rules like this, and response latencies often improve by a factor of two or more. Second, an asymmetry in rule application appears. Initially, there is little difference whether participants are asked to apply the rule in one direction or the other (computing the second time given the first or vice versa). However, if the participants practice rules in only one direction, they become faster in the direction in which they have practiced.
Experiment 1
The first experiment followed a 2-day protocol where participants were trained on the first day and returned the next day for the imaging session. Training involved extensive practice in learning and applying rules uniquely associated with eight different sports. Each rule was created by pairing a day operator (either incrementing or decrementing the day by 1 or 2) with an hour operator (either incrementing or decrementing the hour by 1 or 2) and assigning a unique sport label (e.g., hockey was +2, −1; baseball was −1, −2; and so on). Participants initially learned these associations explicitly and so did not need to infer them, differing from the original behavioral work. Table 1 illustrates the full range of stimulus types presented during the imaging session. The table shows a 2 × 2 within-subject design, where two factors were manipulated: recall demand and rule direction. Recall demand was manipulated by giving participants two types of stimuli: recall and explicit. A recall trial was one where a sport label was presented with a random given day and hour. Using the sport cue, participants had to recall the associated rule and apply the recalled rule to the given day and hour to compute the target response. Explicit trials differed from recall trials, in that they required no recall of a rule. Rather than presenting a sport label to cue the rule in these stimuli, an explicit label like “+2, −1” was used instead, so the requisite rule was explicitly provided.
Table 1.
Typical stimuli presented to participants in Experiments 1 and 2
| Forward | Reverse | |
|---|---|---|
| Explicit | Tuesday 3 | ----- - |
| +2 −1 | +2 −1 | |
| ----- - | Thursday 2 | |
| Recall | Wednesday 5 | ----- - |
| Hockey | Hockey | |
| ----- - | Friday 4 |
Shown are forward and reverse explicit stimuli (top, Experiment 1) and forward and reverse recall stimuli (bottom, both experiments).
The direction manipulation relates to the direction in which a computation was performed, which in turn depended on the direction in which a participant practiced during training. Some participants practiced going from the top to the bottom of the screen (that is, day and hour were displayed across the top of the screen, empty target slots at the bottom), whereas others practiced solving from the bottom to the top of the screen. A reverse trial for someone practiced in the top-to-bottom direction was a stimulus where the bottom, or result, was given, and the participant had to “work backward” to fill in the empty slots given at the top of the screen. Thus, simply noting a presented stimulus was consistent with the direction of the training, indicating the computation should be performed in the usual way (the forward direction). Noting that a stimulus was inconsistent with the training direction indicated the computation should be performed in the reverse direction, where the signs of the rule needed to be inverted before being applied to the presented day and hour.
Results.
Table 2 presents the error and accuracy data for the experiment. Repeated-measures ANOVAs were computed for both error and latency data. There was a main effect of recall on accuracy [F (1,6) = 9.658, P = 0.021, mean squared error (MSE) = 0.015], a marginal effect of direction [F (1,6) = 3.761, P = 0.10, MSE = 0.011], and no recall by direction interaction [F (1,6) = 0.073, MSE = 0.014]. The latency data generally mirrored the error data. There were main effects of recall [F (1,6) = 31.168, P = 0.001, MSE = 225,585] and direction [F (1,6) = 172.414, P = 0.001, MSE = 2,996] but not a significant recall by direction interaction [F (1,6) = 0.531, MSE = 82,856].
Table 2.
Experiment 1: Latency in milliseconds (standard error) and proportion error (standard error) by condition
| Rule type | Direction |
|
|---|---|---|
| Forward | Reverse | |
| Explicit | 3,837 (351) | 4,617 (339) |
| 0.10 (0.041) | 0.19 (0.03) | |
| Recall | 4,760 (291) | 5,698 (322) |
| 0.26 (0.05) | 0.32 (0.056) | |
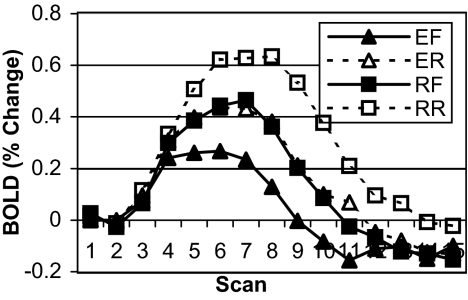
The blood-oxygenation level-dependent (BOLD) response for each trial was computed by using the mean of the first two data scans of the trial as the baseline from which percent change was calculated over the full time course of the trial. The data used in the following analyses were the areas under the BOLD response curves over the first 12 scans for each trial. The assumption in using this measure is that the area under the curve in a particular condition serves as a proxy for the amount of work done by that area during that condition (36). Figs. 2 and 3 show the BOLD data for the PFC and ACC regions, respectively. In the PFC, there was a main effect of recall [explicit rule vs. recall rule; F (1,6) = 9.778, P < 0.02, MSE = 0.1284] and a main effect of direction [forward vs. reverse; F (1,6) = 17.155, P < 0.006, MSE = 0.763]. The recall by direction interaction was not significant. Similarly, in the ACC, there was a main effect of recall [explicit rule vs. recall rule, F (1,6) = 11.324, P < 0.02, MSE = 0.467] and a main effect of direction [F (1,6) = 10.026, P < 0.02, MSE = 0.472]. The recall by direction interaction was not significant.
Fig. 2.
Experiment 1 BOLD data for predefined PFC ROI. Data are plotted as percent change from baseline (mean of scans 1 and 2) over the course of a single trial (15 scans, 18 sec) for four conditions (EF, explicit forward; ER, explicit reverse; RF, recall forward; and RR, recall reverse).
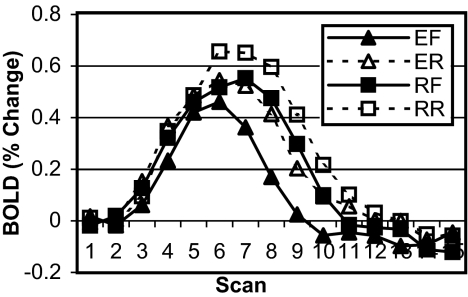
Fig. 3.
Experiment 2 BOLD data for predefined PFC ROI. Data are plotted as percent change from baseline (mean of scans 1 and 2) over the course of a single trial (15 scans, 18 sec) by imaging session and condition (S1, imaging session 1; S2F, imaging session 2 forward rules; S2R, imaging session 2 reverse rules).
This experiment confirmed that both the ACC and PFC responded to the manipulation of retrieval (recall vs. explicit) in the same way that they responded to the manipulation of rule direction (forward vs. reverse). The more difficult conditions under both manipulations required participants to engage in extra steps of recall, increasing retrieval demands that impacted the PFC and increasing control demands that impacted the ACC.
Experiment 2
The previous experiment illustrated a situation were the PFC and ACC responded similarly. However, the response of these two regions was attributed to different factors. In the case of the PFC, it was the requirement for an extra retrieval, and in the case of the ACC, it was the requirement of extra control for that retrieval. According to this analysis, the expectation is that any complexity manipulation requiring an extra step of retrieval will produce increased responses in both regions. However, if this analysis is correct, it should be possible to dissociate the two areas. One should be able to get a differential response in the PFC but not the ACC by manipulating the difficulty of a retrieval step (through practice) rather than the number of retrieval steps. Conversely, one should be able to get a differential response in the ACC but not the PFC by introducing a control step that does not require extra retrieval.
The second experiment used a multiday protocol where two event-related imaging sessions were conducted, so that activity at the beginning and end of practice with the task could be compared. Two manipulations were used to produce different directions of effects in the ACC and the PFC. First, the experiment attempted to reduce the PFC response by giving 2 days of extensive practice between imaging sessions. The practice should speed up retrieval of the relevant information but not change the number of steps of processing required. Second, the experiment attempted to increase the number of steps of processing on the last day without increasing the number of steps of retrieval. This was achieved by introducing a forward/reverse direction manipulation on the last day. This would require an extra decision step to decide in which direction to apply the rule, but we attempted to design the materials so an extra retrieval step would not be required.
In the previous experiment, participants were initially given the rule for each sport in explicit form, like “+2, −1.” This representation constrained the rules to be inherently directional and so resulted in an extra step of inversion when working in the reverse direction. The current experiment modified that procedure somewhat to make the material initially neutral to direction. Participants were taught the material in a manner closer to the original behavioral experiments, where on the first day, they memorized six different sports facts of the form “Hockey is played on Tuesday at 3:00 p.m. and Thursday at 2:00 p.m.” In this form, knowledge is not given an explicit direction, like “+2, −1,” as it was given in the first experiment. Therefore, it is equally appropriate for application in either direction, and participants were free to extract a representation of their choosing in relating the timing of events for each sport. Of course, as participants practiced the material, they probably extracted some form of directional rule (42); however, the hope was that their representation would not require the inversion of an explicit rule.
To summarize the expectations of the two manipulations, extensive practice should reduce retrieval time. Introducing the directionality manipulation on the last day should yield an extra step of decision making in which participants had to note in which direction a rule should be applied. Thus, the strong predictions were that the PFC should show a reduced response from the beginning to the end of practice, reflecting faster retrieval, whereas the ACC should show an increased response on the final day, reflecting increased demand from extra decision making.
Results.
The accuracy and latency effects are shown in Table 3. Repeated-measures ANOVAs were computed for both error data and latency data by using trial type as a factor [imaging session 1 (S1), imaging session 2 forward (S2F), imaging session 2 reverse (S2R)]. Only correct trials were included in the analysis of the latency data. There were main effects of trial type for both proportion error [F (2,22) = 11.61, P < 0.001, MSE = 0.003] and latency [F (2,22) = 14.54, P < 0.001, MSE = 2,834,573]. In terms of these behavioral measures, S1 forward rules were the most difficult, followed by S2R and finally S2F.
Table 3.
Experiment 2: Latency in milliseconds (standard error) and proportion error (standard error) by imaging session and condition
| Session/condition | Latency | Proportion error |
|---|---|---|
| S1 | 9,073 (1093) | 0.19 (0.031) |
| S2F | 5,377 (650) | 0.09 (0.011) |
| S2R | 6,986 (808) | 0.11 (0.013) |
S1, imaging session 1; S2F, imaging session 2 forward; S2R, imaging session 2 reverse.
Fig. 4 shows the BOLD data for the PFC region. The main expectation of a reduction in PFC activity due to increased retrieval speed with practice was confirmed; the magnitude of BOLD response for forward rules was smaller during session 2 than session 1 [F (1,11) = 8.674, P < 0.013, MSE = 0.662]. Reverse rules elicited a stronger response than forward rules on session 2, but this was only marginally significant [F (1,11) = 4.303, P < 0.062, MSE = 0.922]. This directional effect replicates the first experiment.
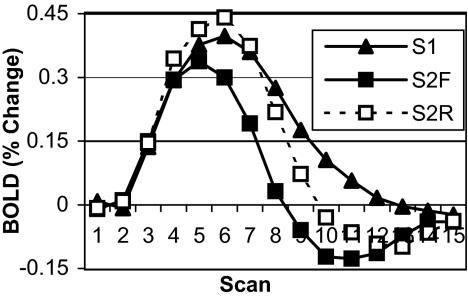
Fig. 4.
Experiment 2 BOLD data for predefined ACC ROI. Data are plotted as percent change from baseline (mean of scans 1 and 2) over the course of a single trial (15 scans, 18 sec) by imaging session and condition (S1, imaging session 1; S2F, imaging session 2 forward rules; S2R, imaging session 2 reverse rules).
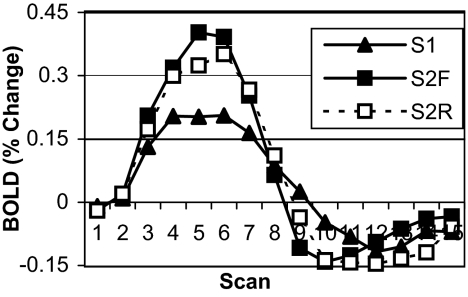
Fig. 5 shows the BOLD data for the ACC region. The main expectation of a greater ACC response in the final session because of increased control demands was also confirmed; ACC activity for session 2 forward rules was significantly greater than for session 1 forward rules [F (1,11) = 10.027, P < 0.009, MSE = 0.082]. This result establishes a clear dissociation between the PFC and the ACC. There was not a significant difference between forward and reverse rules during session 2 [F (1,11) = 0.077, MSE = 0.288]. The lack of an effect of direction contrasts with the effect of direction on the ACC in the first experiment, consistent with the experimental goal of eliminating an extra step of explicit rule inversion for reverse rules.
Fig. 5.
Experiment 2 BOLD data for predefined ACC ROI. Data are plotted as percent change from baseline (mean of scans 1 and 2) over the course of a single trial (15 scans, 18 sec) by imaging session and condition (S1, imaging session 1; S2F, imaging session 2 forward rules; S2R, imaging session 2 reverse rules).
To further corroborate the notion that the extra decision-making step during the final imaging session in fact had an impact cognitively, the behavioral data from session 2 forward rules were compared to the practice data in the laboratory on the previous day. Indeed, the error rate during the last imaging session was significantly greater than the immediately previous nonimaging practice session [0.089 vs. 0.048, F (1,11) = 19.269, P < 0.001, MSE = 0.001], and response latencies were significantly longer [5,377 vs. 4,591 msec, F (1,11) = 5.278, P < 0.05, MSE = 701,961]. These differences reflect the cost of making the extra decision step during the final imaging session.
Conclusions
The two experiments have used the same basic paradigm to produce strikingly different patterns of results in the PFC and the ACC. In Experiment 1, where the manipulations introduced extra steps of retrieval, there were parallel effects in the two regions. This is predicted by a model in which additional retrievals require additional control states. In contrast, Experiment 2 showed the PFC and the ACC responded in almost opposite manners, as revealed by the contrast across sessions for forward rules. The PFC reduced its response in session 2, reflecting the faster retrieval, and the ACC increased its response, reflecting the increased task-related decision-making demand.
Although we have never before produced a demonstration of opposite response in the PFC and the ACC, these results are consistent with the general effects in our laboratory. In two tasks [one algebra (22) and one an algebra isomorph (19)], the number of steps of algebraic transformation increased the response in both the PFC and the ACC, whereas practice produced a reduction in PFC activity but had no effect on ACC activity. Each step of algebraic transformation required both extra control steps and retrieval of extra knowledge to perform the transformation. However, practice only sped up the retrieval process and had no effect on the number of control steps, because the participant still had to go through the same mental transformations.
At a general level, the current work has demonstrated several important points. The decrease in PFC activity over the course of learning is a typical result found in the literature, and this effect is attributed to the increased efficiency of the retrieval process (3). On the other hand, the results for the ACC could be viewed as a relatively uncommon result, because its activity increased after extensive practice. However, the proposed model of the role of ACC in control explains this result, because an extra control step was required during the final session. Finally, it is notable that the pattern of activity shown by the ACC in the second experiment is not consistent with a general view where increased activity reflects increases in difficulty, error rates, or latency measures. Indeed, ACC activity was greatest when error rates and latencies were smallest.
Methods
Participants.
Seven (two male) right-handed native English-speaking Carnegie Mellon University (CMU) graduate and undergraduate students enrolled in Experiment 1. Participant ages ranged from 22 to 34 years of age. Twelve (three male) right-handed native English-speaking CMU graduate and undergraduate students enrolled in Experiment 2. Participant ages ranged from 18 to 24 years of age. Institutional Review Board approval was obtained from both CMU and the University of Pittsburgh. All participants gave informed consent in accordance with CMU and University of Pittsburgh guidelines.
Experiment 1.
Two-day protocol.
The stimulus structure in this experiment was derived from the stimuli reported in ref. 41. There were underlying arithmetic relationships for eight sports between the first and second event times, as described earlier for Experiment 1.
Day 1.
Participants memorized and practiced associations of sport label to arithmetic operators for each of eight unique sports. There were eight blocks of training. Within each block, each of the eight sports was practiced six times for a total of 48 trials per block yielding a total of 384 practice trials. The given day and time presented with a sport during a particular trial were selected randomly. Trials during the first two blocks presented the underlying rule with the sport label. Participants were told they needed to memorize the associations, which were not presented in subsequent blocks. Four of the participants practiced in a top-to-bottom screen presentation, the rest in bottom-to-top screen presentation.
Day 2: Imaging session.
Before imaging, participants were instructed that they would be presented with a mixture of trials, including those like they had been practicing, explicit trials, and reverse trials (see Experiment 1). Before imaging, they performed two practice blocks identical in structure to the training blocks on day 1. Within a block, each of the eight sports was practiced six times for a total of 48 trials per block. Next, participants practiced two blocks of explicit rules identical in structure to the blocks presented on day 1, with the difference that the sport label was replaced by the explicit rule. Each of the eight rules was practiced six times in a block for a total of 48 trials per block. After this practice, participants took part in the imaging phase of the experiment.
Imaging was event-related. Each trial lasted for 18 sec and was synchronized with scan onset. When a response was given, the display transitioned into a fixation stimulus that remained on for the remainder of the 18 sec. Participants responded with a right-handed five-button response glove. When the target response was a day of the week, the mapping from thumb through pinky finger was Monday through Friday. Similarly, when the response target was a digit, the mapping from thumb through pinky finger was digits one through five. Each response consisted of two key presses; by convention, the first indicated the target day and the second indicated the target hour.
Functional images were acquired with a conventional 3.0-T GE (General Electric) Signa whole-body scanner using a standard RF head coil. Twenty-seven oblique axial slices (3.20-mm thick, 3.125-mm2 in-plane resolution) were acquired parallel to the anterior commissure–posterior commissure (AC-PC) line, with the middle of the fifth slice from the bottom through the AC-PC line (a total of 86.4 mm of brain coverage). Functional images were acquired by using a two-shot T2*-weighted reverse spiral scan pulse sequence [repetition time (TR) = 1,200 msec, echo time (TE) = 34 msec, field of view = 20 cm, flip angle = 70°; ref. 43]. Scanning was event-related, with image acquisition synchronized to stimulus onset, such that 15 volumes, each containing 27 slices, were acquired during each 18-sec trial. Anatomical scans (36 slices) were acquired by using a standard T1-weighted pulse sequence, with the middle of the 15th slice from the bottom through the AC-PC line. Images were then coregistered to a common reference structural MRI scan by means of a 12-parameter algorithm (44) automatic image registration (AIR) and smoothed with an 8-mm full-width-half-maximum 3D Gaussian filter to accommodate individual differences in anatomy.
Participants performed 8–10 blocks of 20 trials each during the imaging session. In eight trials within a block, the participant simply copied the givens as a response and served to establish baseline measures. Four trials were fixation trials, in which the participant could simply rest. There were eight critical trials in each block consisting of two observations of each of the four conditions in Table 1. Each imaging trial lasted for 18 sec and was synchronized with scan onset. Participants were given 8.4 sec to completely respond. If participants responded correctly, the display remained in place with target items filled in for 1.2 sec and then transitioned into a fixation stimulus that remained on for the remainder of the 18 sec. If they did not respond in the allotted time, the display flashed red for 1.2 sec and then was updated to show the fixation stimulus for the remainder of the 18 sec. Similarly, any time the participant responded incorrectly, the display flashed red for 1.2 sec and then transitioned into the fixation display for the remainder of the trial. Both of these error trial types were ignored in subsequent analyses.
Experiment 2.
Five-day protocol.
The stimulus structure in this experiment was similar to that presented in the first experiment. For six sports, there were underlying arithmetic relationships between two event times.
Day 1.
Participants memorized six different sport-scheduling facts of the form “Hockey is done on Tuesday at 3:00 p.m. and Thursday at 2:00 p.m.” There were 16 memorization blocks where only the sport label was given, and participants had to reproduce the 2 days and 2 hours from the original fact. Each of the six sports was presented three times per block for a total of 18 trials per block, yielding 3 × 16 = 48 practice trials for each fact at the end of the memorization.
Day 2: Imaging session 1.
Before imaging, participants were told there was an underlying relationship unique to each sport relating the days and hours for that sport. Participants were instructed that they would be given partially complete stimuli containing a random day and a random hour. To solve each problem, they would have to remember the memorized example for the presented sport, infer the underlying rule that related the timings of the events, and use that inferred relationship to compute the target response. There were eight 9-min scanning blocks (detailed scanning procedure provided after the day 5 protocol below). Each stimulus was randomly constructed by using one of the six available sports, the days Monday through Friday, and the digits one through five. The presentation rate was self-paced, and the number of trials per block depended on how quickly participants solved problems.
Days 3 and 4.
There were 12 blocks of training on each day using randomly constructed stimuli, as on day 2. Within a block, three of the sports were practiced 10 times each, and the remaining three sports were practiced two times each. At the end of the two training sessions, half of the sports received 240 trials of practice, whereas the other half received 48 trials of practice (the frequency manipulation was collapsed over in subsequent analyses because its effects were weak).
Day 5: Imaging session 2.
Before imaging, participants were instructed that half of the trials would be presented in the same manner as they had been practicing in the previous training sessions, whereas the other half would be presented in the opposite direction (as described earlier in Experiment 2), where one needed to work backward to compute the target response. There were eight 9-min blocks. Stimuli were generated randomly as on day 2, and forward and reverse trials were randomly mixed over the course of each block, each occurring half the time. As in the first imaging phase, presentations were self-paced.
The scanning procedure on days 2 and 5 was identical. Event-related functional images were acquired by using gradient echo-planar image acquisition on a Siemens (Iselin, NJ) 3-T Allegra Scanner with a standard RF head coil. Imaging parameters were repetition time = 1,500 msec, echo time = 25 msec, RF flip angle = 55°, field of view = 200 mm, matrix size = 64 × 64 (3.125 × 3.125-mm in-plane resolution per voxel), slice thickness = 3.2 mm, slice gap = 0 mm, and 29 oblique axial slices (parallel to the AC-PC line) per volume scan with the AC-PC at slice 22 from the superior. Anatomical scans (37 slices) were acquired by using a standard T2-weighted spin-echo pulse sequence, with the middle of the 26th slice from the superior through the AC-PC line. All images were coregistered to a common reference structural MRI scan by means of a 12-parameter automatic image registration algorithm (44) automatic image registration (AIR) and smoothed with an 6-mm full-width-half-maximum 3D Gaussian filter to accommodate individual differences in anatomy.
Scanning blocks were 9 min in length. The task was self-paced, so the number of trials per block was variable. After a response was indicated, a fixation was displayed for eight scans (12 sec) plus an additional amount of time (less than repetition time = 1,500 msec), such that the onset of the next stimulus occurred simultaneously with the beginning of the next full volume scan.
Participants were given 30 sec to completely respond. If participants responded correctly, the display remained in place with target items filled in for 1.5 sec and then transitioned into a fixation stimulus that remained on for the remainder of the trial (≈12 sec, as described above). If they did not respond in the allotted time, the display flashed red for 1.5 sec, then updated to show the fixation stimulus for the remainder of the trial. Similarly, any time the participant responded incorrectly, the display flashed red for 1.5 sec and then transitioned into the fixation display for the remainder of the trial. Both of these error trial types were ignored in subsequent analyses.
Acknowledgments
We thank Andrea Stocco and Jennifer L. Ferris for providing comments on previous versions of this manuscript. This research was supported by National Science Foundation Grant 0336585, Research on Learning and Education: Tracking the Course of Mathematical Learning (to J.R.A.).
Abbreviations
- ACC
anterior cingulate cortex
- PFC
prefrontal cortex
- fMRI
functional MRI
- ROI
region of interest
- MSE
mean squared error
- BOLD
blood-oxygenation level-dependent
- AC-PC
anterior commissure–posterior commissure
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Cohen J. D., Botvinick M., Carter C. S. Nat. Neurosci. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- 2.Ridderinkhof K. R., van den Wildenberg W. P., Segalowitz S. J., Carter C. S. Brain Cognit. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Kelly A. M. C., Garavan H. Cereb. Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- 4.Hill N. M., Schneider W. In: Cambridge Handbook of Expertise and Expert Performance. Ericsson K. A., Charness N., Feltovich P., Hoffman R., editors. New York: Cambridge Univ. Press; 2006. in press. [Google Scholar]
- 5.Ridderinkhof K. R., Ullsperger M., Crone E. A., Nieuwenhuis S. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 6.Bush G., Luu P., Posner M. I. Trends Cognit. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 7.Schneider W., Chein J. M. Cognit. Sci. 2003;27:525–559. [Google Scholar]
- 8.Posner M. I., Dehaene S. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 9.D’Esposito M., Detre J. A., Alsop D. C., Shin R. K., Atlas S., Grossman M. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- 10.Posner M. I., DiGirolamo G. J. In: The Attentive Brain. Parasuraman R., editor. Cambridge, MA: MIT Press; 1998. pp. 401–423. [Google Scholar]
- 11.Falkenstein M., Hohnsbein J., Hoormann J. Electroencephalogr. Clin. Neurophysiol. Suppl. 1995;44:287–296. [PubMed] [Google Scholar]
- 12.Dehaene S., Posner M. I., Tucker D. M. Psychol. Sci. 1994;5:303–305. [Google Scholar]
- 13.Paus T., Koski L., Caramanos Z., Westbury C. NeuroReport. 1998;9:R37–R47. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 14.Carter C. S., Macdonald A. M., Botvinick M., Ross L. L., Stenger V. A., Noll D., Cohen J. D. Proc. Natl. Acad. Sci. USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botvinick M., Braver T. S., Yeung N., Ullsperger M., Carter C. S., Cohen J. D. In: Cognitive Neuroscience of Attention. Posner M. I., editor. New York: Guilford; 2004. p. xiii. [Google Scholar]
- 16.MacDonald A. W., III, Cohen J. D., Stenger V. A., Carter C. S. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 17.Van Veen V., Carter C. S. J. Cognit. Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- 18.van Veen V., Cohen J. D., Botvinick M. M., Stenger V. A., Carter C. S. NeuroImage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- 19.Anderson J. R. In: Integrated Models of Cognitive Systems. Gray W. D., editor. New York: Oxford Univ. Press; 2006. in press. [Google Scholar]
- 20.van Veen V., Carter C. S. NeuroImage. 2005;27:497–504. doi: 10.1016/j.neuroimage.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Maril A., Wagner A. D., Schacter D. L. Neuron. 2001;31:653–660. doi: 10.1016/s0896-6273(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 22.Anderson J. R. Cognit. Sci. 2005;29:313–341. doi: 10.1207/s15516709cog0000_22. [DOI] [PubMed] [Google Scholar]
- 23.Anderson J. R., Bothell D., Byrne M. D., Douglass S., Lebiere C., Qin Y. Psychol. Rev. 2004;111:1036–1060. doi: 10.1037/0033-295X.111.4.1036. [DOI] [PubMed] [Google Scholar]
- 24.Cabeza R., Nyberg L. J. Cognit. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 25.Duncan J., Owen A. M. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 26.Bush G., Whalen P. J., Rosen B. R., Jenike M. A., McInerney S. C., Rauch S. L. Hum. Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paus T., Petrides M., Evans A. C., Meyer E. J. Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- 28.Taylor S. F., Kornblum S., Lauber E. J., Minoshima S., Koeppe R. A. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- 29.Rugg M. D., Henson R. N. A. In: The Cognitive Neuroscience of Memory: Encoding and Retrieval. Parker A., Wilding E., Bussey T., editors. Hove, U.K.: Psychology Press; 2002. pp. 3–37. [Google Scholar]
- 30.Buckner R. L., Kelley W. M., Petersen S. E. Nat. Neurosci. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- 31.Cabeza R., Dolcos F., Graham R., Nyberg L. NeuroImage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher P. C., Henson R. N. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 33.Lepage M., Ghaffar O., Nyberg L., Tulving E. Proc. Natl. Acad. Sci. USA. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner A. D., Maril A., Bjork R. A., Schacter D. L. NeuroImage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- 35.Wagner A. D., Pare-Blagoev E. J., Clark J., Poldrack R. A. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- 36.Anderson J. R., Qin Y., Stenger V. A., Carter C. S. J. Cognit. Neurosci. 2004;16:637–653. doi: 10.1162/089892904323057353. [DOI] [PubMed] [Google Scholar]
- 37.Qin Y., Carter C. S., Silk E. M., Stenger V. A., Fissell K., Goode A., Anderson J. R. Proc. Natl. Acad. Sci. USA. 2004;101:5686–5691. doi: 10.1073/pnas.0401227101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin Y., Sohn M. H., Anderson J. R., Stenger V. A., Fissell K., Goode A., Carter C. S. Proc. Natl. Acad. Sci. USA. 2003;100:4951–4956. doi: 10.1073/pnas.0431053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sohn M. H., Goode A., Stenger V. A., Carter C. S., Anderson J. R. Proc. Natl. Acad. Sci. USA. 2003;100:7412–7417. doi: 10.1073/pnas.0832374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sohn M. H., Goode A., Stenger V. A., Jung K. J., Carter C. S., Anderson J. R. NeuroImage. 2005;25:21–33. doi: 10.1016/j.neuroimage.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Anderson J. R., Fincham J. M. J. Exp. Psychol. Learn. Mem. Cognit. 1994;20:1322–1340. doi: 10.1037//0278-7393.20.6.1322. [DOI] [PubMed] [Google Scholar]
- 42.Anderson J. R., Fincham J. M., Douglass S. J. Exp. Psychol. Learn. Mem. Cognit. 1997;23:932–945. doi: 10.1037//0278-7393.23.4.932. [DOI] [PubMed] [Google Scholar]
- 43.Noll D. C., Cohen J. D., Meyer C. H., Schneider W. J. Magn. Reson. Imag. 1995;5:49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- 44.Woods R. P., Cherry S. R., Mazziotta J. C. J. Comput. Assist. Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]