Abstract
A single intraperitoneal injection (0.6 ml/kg) of dimethoxyethyl phthalate (DMEP) was given to groups of Wistar strain rats on day 10, 11, 12, 13 or 14 of gestation. Control rats received 0.6 ml/kg of physiological saline intraperitoneally. In phthalate-treated rats, embryopathy was manifested by a high incidence (12-79%) of fetal deaths and fetal resorptions. Fetotoxic effects were expressed by a significant reduction in fetal weights. Hydrocephalus interna, a congenital malformation of the brain, was caused by DMEP. Congenital skeletal deformities (66-96%), with multiple skeletal (14-64%) and appendicular malformations (25-57%), were also induced by DMEP. Control rats exhibited no congenital malformations of the brain and no appendicular or multiple skeletal deformities. DMEP caused a significant decrease in the zinc content of the fetus. Fetoplacental metabolism 1 and 4 hr after intravenous administration of 14C-DMEP suggested rapid transfer of the parent compound to the fetus across the placenta and that DMEP is a teratogenic moiety. The possible role of zinc in phthalate-induced teratogenesis in rats is also discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeloye A., Warkany J. Experimental congenital hydrocephalus. A review with special consideration of hydrocephalus produced by zinc deficiency. Childs Brain. 1976;2(6):325–360. [PubMed] [Google Scholar]
- Autian J. Toxicity and health threats of phthalate esters: review of the literature. Environ Health Perspect. 1973 Jun;4:3–26. doi: 10.1289/ehp.73043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower R. K., Haberman S., Minton P. D. Terotogenic effects in the chick embryo caused by esters of phthalic acid. J Pharmacol Exp Ther. 1970 Feb;171(2):314–324. [PubMed] [Google Scholar]
- Burch R. E., Sullivan J. F. Clinical and nutritional aspects of zinc deficiency and excess. Med Clin North Am. 1976 Jul;60(4):675–685. doi: 10.1016/s0025-7125(16)31852-1. [DOI] [PubMed] [Google Scholar]
- Calley D., Autian J., Guess W. L. Toxicology of a series of phthalate esters. J Pharm Sci. 1966 Feb;55(2):158–162. doi: 10.1002/jps.2600550206. [DOI] [PubMed] [Google Scholar]
- Cater B. R., Cook M. W., Gangolli S. D., Grasso P. Studies on dibutyl phthalate-induced testicular atrophy in the rat: effect on zinc metabolism. Toxicol Appl Pharmacol. 1977 Sep;41(3):609–618. doi: 10.1016/s0041-008x(77)80014-8. [DOI] [PubMed] [Google Scholar]
- Chu I., Villeneuve D. C., Secours V., Franklin C., Rock G., Viau A. Metabolism and tissue distribution of mono-2-ethylhexyl phthalate in the rat. Drug Metab Dispos. 1978 Mar-Apr;6(2):146–149. [PubMed] [Google Scholar]
- Daniel J. W., Bratt H. The absorption, metabolism and tissue distribution of di(2-ethylhexyl)phthalate in rats. Toxicology. 1974 Mar;2(1):51–65. doi: 10.1016/0300-483x(74)90042-0. [DOI] [PubMed] [Google Scholar]
- Daniel J. W. Toxicity and metabolism of phthalate esters. Clin Toxicol. 1978;13(2):257–268. doi: 10.3109/15563657808988236. [DOI] [PubMed] [Google Scholar]
- Dillingham E. O., Autian J. Teratogenicity, mutagenicity, and cellular toxicity of phthalate esters. Environ Health Perspect. 1973 Jan;3:81–89. doi: 10.1289/ehp.730381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin N. H., Baros N. A., Cox R. T., King T. M. Implantation and fetal survival in the rat as affected by intrauterine injection of normal sterile saline. Biol Reprod. 1979 Aug;21(1):47–52. doi: 10.1095/biolreprod21.1.47. [DOI] [PubMed] [Google Scholar]
- FEASTER J. P., HANSARD S. L., McCALL J. T., DAVIS G. K. Absorption, deposition and placental transfer of zinc65 in the rat. Am J Physiol. 1955 May;181(2):287–290. doi: 10.1152/ajplegacy.1955.181.2.287. [DOI] [PubMed] [Google Scholar]
- Foster P. M., Thomas L. V., Cook M. W., Gangolli S. D. Study of the testicular effects and changes in zinc excretion produced by some n-alkyl phthalates in the rat. Toxicol Appl Pharmacol. 1980 Jul;54(3):392–398. doi: 10.1016/0041-008x(80)90165-9. [DOI] [PubMed] [Google Scholar]
- Guess W. L., Haberman S. Toxicity profiles of vinyl and polyolefinic plastics and their additives. J Biomed Mater Res. 1968 Sep;2(3):313–335. doi: 10.1002/jbm.820020304. [DOI] [PubMed] [Google Scholar]
- Hillman L. S., Goodwin S. L., Sherman W. R. Identification and measurement of plasticizer in neonatal tissues after umbilical catheters and blood products. N Engl J Med. 1975 Feb 20;292(8):381–386. doi: 10.1056/NEJM197502202920801. [DOI] [PubMed] [Google Scholar]
- Hurley L. S., Swenerton H. Congenital malformations resulting from zinc deficiency in rats. Proc Soc Exp Biol Med. 1966 Dec;123(3):692–696. doi: 10.3181/00379727-123-31578. [DOI] [PubMed] [Google Scholar]
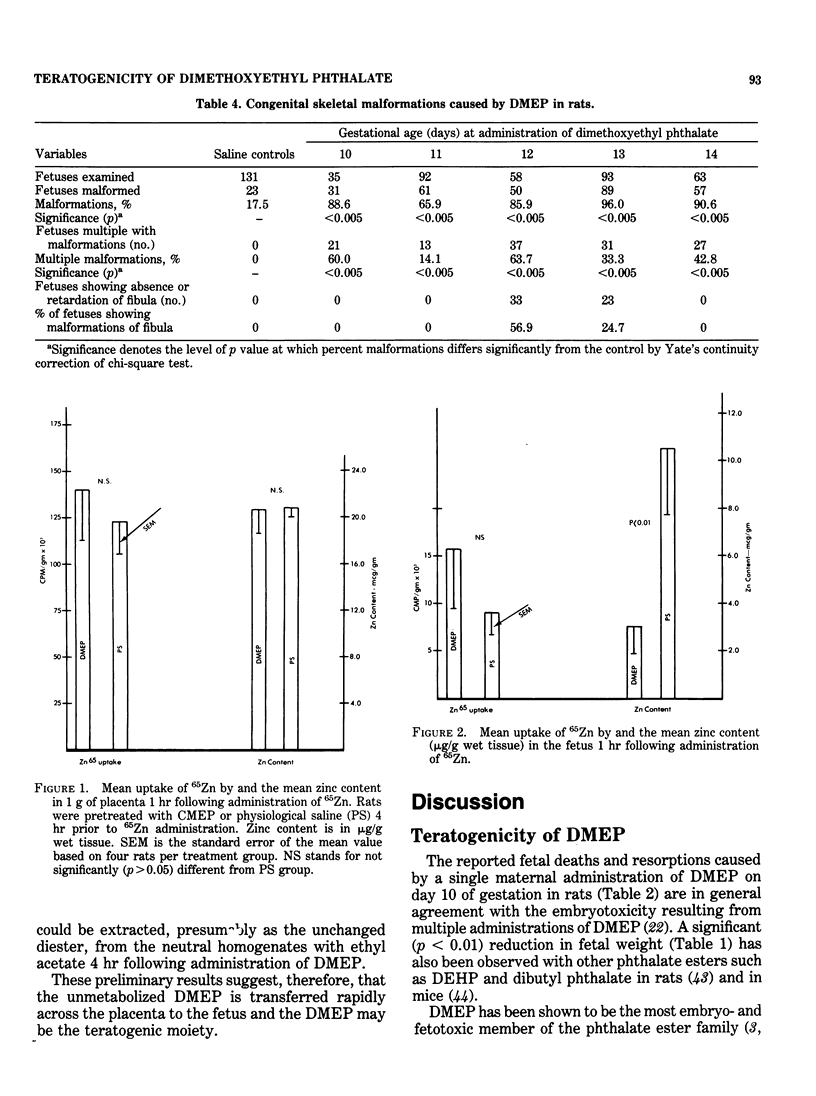
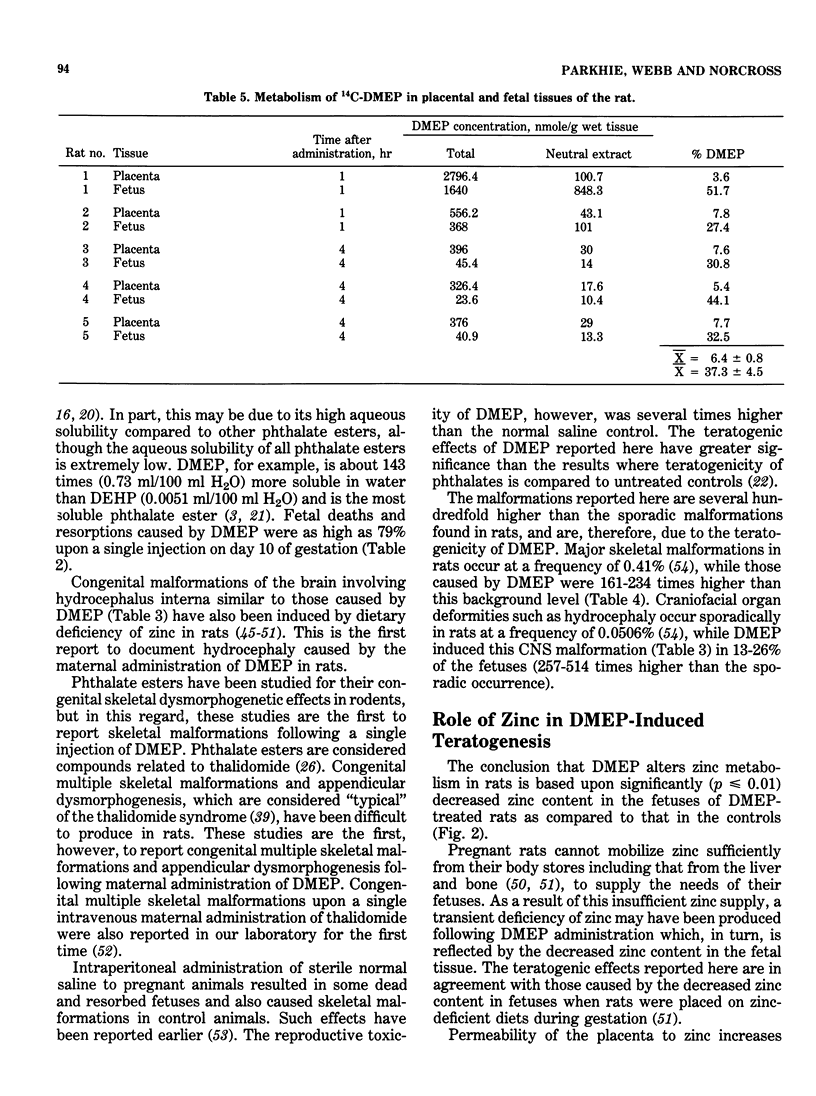
- Hurley L. S., Swenerton H. Lack of mobilization of bone and liver zinc under teratogenic conditions of zinc deficiency in rats. J Nutr. 1971 May;101(5):597–603. doi: 10.1093/jn/101.5.597. [DOI] [PubMed] [Google Scholar]
- Ikeda G. J., Sapienza P. P., Couvillion J. L., Farber T. M., Smith C. P., Inskeep P. B., Marks E. M., Cerra F. E., van Loon E. J. Distribution and excretion of two phthalate esters in rats, dogs and miniature pigs. Food Cosmet Toxicol. 1978 Oct;16(5):409–413. doi: 10.1016/s0015-6264(78)80257-0. [DOI] [PubMed] [Google Scholar]
- Jackson A. J., Schumacher H. J. The teratogenic activity of a thalidomide analogus EM12 in rats on a low-zinc diet. Teratology. 1979 Jun;19(3):341–344. doi: 10.1002/tera.1420190310. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Rubin R. J. Migration of a phthalate ester plasticizer from polyvinyl chloride blood bags into stored human blood and its localization in human tissues. N Engl J Med. 1972 Nov 30;287(22):1114–1118. doi: 10.1056/NEJM197211302872203. [DOI] [PubMed] [Google Scholar]
- Jones A. E., Kahn R. H., Groves J. T., Napier E. A., Jr Phthalate ester toxicity in human cell cultures. Toxicol Appl Pharmacol. 1975 Feb;31(2):283–289. doi: 10.1016/0041-008x(75)90163-5. [DOI] [PubMed] [Google Scholar]
- Lawrence W. H. Phthalate esters: the question of safety. Clin Toxicol. 1978;13(1):89–139. doi: 10.3109/15563657808988230. [DOI] [PubMed] [Google Scholar]
- Lawrence W. H., Tuell S. F. Phthalate esters: the question of safety--an update. Clin Toxicol. 1979;15(4):447–466. doi: 10.3109/15563657908989899. [DOI] [PubMed] [Google Scholar]
- Mambidge K. M., Neldner K. H., Walravens P. A. Letter: Zinc,acrodermatitis enteropathica, and congenital malformations. Lancet. 1975 Mar 8;1(7906):577–578. doi: 10.1016/s0140-6736(75)91601-3. [DOI] [PubMed] [Google Scholar]
- Metcalf R. L., Booth G. M., Schuth C. K., Hansen D. J., Lu P. Y. Uptake and fate of Di-2-ethylhexyl phthalate in aquatic organisms and in a model ecosystem. Environ Health Perspect. 1973 Jun;4:27–34. doi: 10.1289/ehp.730427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A. K. Problems associated with the screening of drugs for possible teratogenic activity. Exp Embryol Teratol. 1974;1(0):16–33. [PubMed] [Google Scholar]
- Peakall D. B. Phthalate esters: Occurrence and biological effects. Residue Rev. 1975;54:1–41. doi: 10.1007/978-1-4612-9857-1_1. [DOI] [PubMed] [Google Scholar]
- Rall D. P. The invisible pollution. N Engl J Med. 1972 Nov 30;287(22):1146–1147. doi: 10.1056/NEJM197211302872213. [DOI] [PubMed] [Google Scholar]
- STAPLES R. E., SCHNELL V. L. REFINEMENTS IN RAPID CLEARING TECHNIC IN THE KOH-ALIZARIN RED S METHOD FOR FETAL BONE. Stain Technol. 1964 Jan;39:61–63. [PubMed] [Google Scholar]
- Schulz C. O., Rubin R. J. Distribution, metabolism, and excretion of di-2-ethylhexyl phthalate in the rat. Environ Health Perspect. 1973 Jan;3:123–129. doi: 10.1289/ehp.7303123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher H., Blake D. A., Gurian J. M., Gillette J. R. A comparison of the teratogenic activity of thalidomide in rabbits and rats. J Pharmacol Exp Ther. 1968 Mar;160(1):189–200. [PubMed] [Google Scholar]
- Shibko S. I., Blumenthal H. Toxicology of phthalic acid esters used in food-packaging material. Environ Health Perspect. 1973 Jan;3:131–137. doi: 10.1289/ehp.7303131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota K., Chou M. J., Nishimura H. Embryotoxic effects of di-2-ethylhexyl phthalate (DEHP) and di-n-buty phthalate (DBP) in mice. Environ Res. 1980 Jun;22(1):245–253. doi: 10.1016/0013-9351(80)90136-x. [DOI] [PubMed] [Google Scholar]
- Singh A. R., Lawrence W. H., Autian J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J Pharm Sci. 1975 Aug;64(8):1347–1350. doi: 10.1002/jps.2600640819. [DOI] [PubMed] [Google Scholar]
- Singh A. R., Lawrence W. H., Autian J. Mutagenic and antifertility sensitivities of mice to di-2-ethylhexyl phthalate (DEHP) and dimethoxyethyl phthalate (DMEP). Toxicol Appl Pharmacol. 1974 Jul;29(1):35–46. doi: 10.1016/0041-008x(74)90159-8. [DOI] [PubMed] [Google Scholar]
- Singh A. R., Lawrence W. H., Autian J. Teratogenicity of phthalate esters in rats. J Pharm Sci. 1972 Jan;61(1):51–55. doi: 10.1002/jps.2600610107. [DOI] [PubMed] [Google Scholar]
- Swenerton H., Hurley L. S. Teratogenic effects of a chelating agent and their prevention by zinc. Science. 1971 Jul 2;173(3991):62–64. doi: 10.1126/science.173.3991.62. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Adachi T., Takahashi T., Yamaha T. Biochemical studies on phthalic esters I. Elimination, distribution and metabolism of di-(2-ethylhexyl)phthalate in rats. Toxicology. 1975 May;4(2):253–264. doi: 10.1016/0300-483x(75)90105-5. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Darby T. D., Wallin R. F., Garvin P. J., Martis L. A review of the biological effects of di-(2-ethylhexyl) phthalate. Toxicol Appl Pharmacol. 1978 Jul;45(1):1–27. doi: 10.1016/0041-008x(78)90024-8. [DOI] [PubMed] [Google Scholar]
- Verrett M. J., Mutchler M. K., Scott W. F., Reynaldo E. F., McLaughlin J. Teratogenic effects of captan and related compounds in the developing chicken embryo. Ann N Y Acad Sci. 1969;160(1):334–343. doi: 10.1111/j.1749-6632.1969.tb15853.x. [DOI] [PubMed] [Google Scholar]
- Warkany J., Petering H. G. Congenital malformations of the brain produced by short zinc deficiencies in rats. Am J Ment Defic. 1973 Mar;77(5):645–653. [PubMed] [Google Scholar]
- Warkany J., Petering H. G. Congenital malformations of the central nervous system in rats produced by maternal zinc deficiency. Teratology. 1972 Jun;5(3):319–334. doi: 10.1002/tera.1420050307. [DOI] [PubMed] [Google Scholar]
- Williams D. T., Blanchfield B. J. The retention, distribution, excretion, and metabolism of dibutyl phthalate-7-14 C in the rat. J Agric Food Chem. 1975 Sep-Oct;23(5):854–858. doi: 10.1021/jf60201a047. [DOI] [PubMed] [Google Scholar]


