Abstract
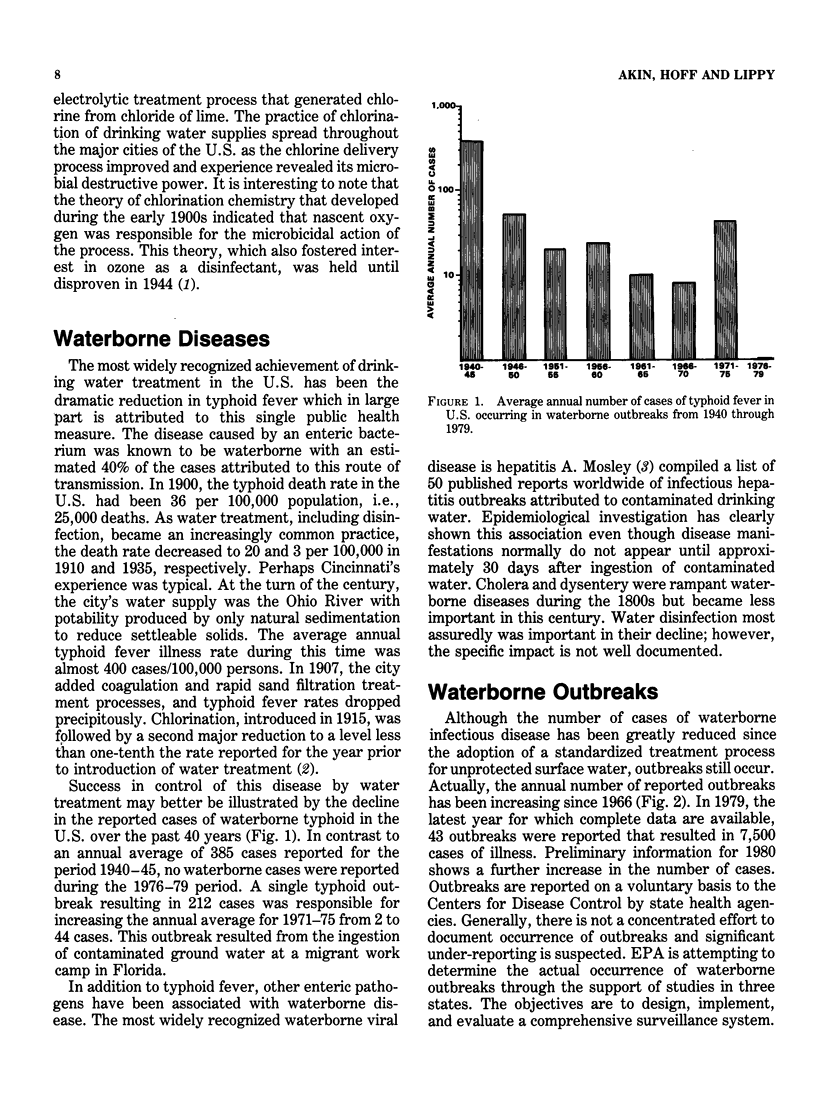
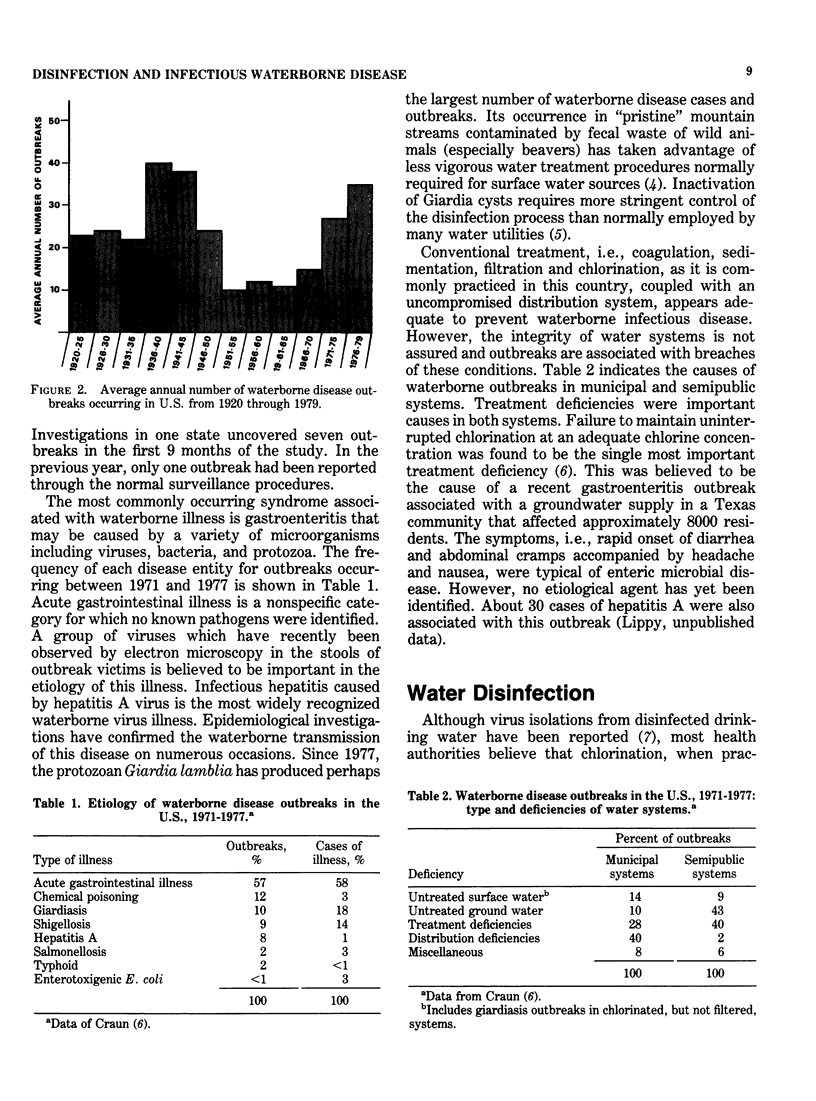
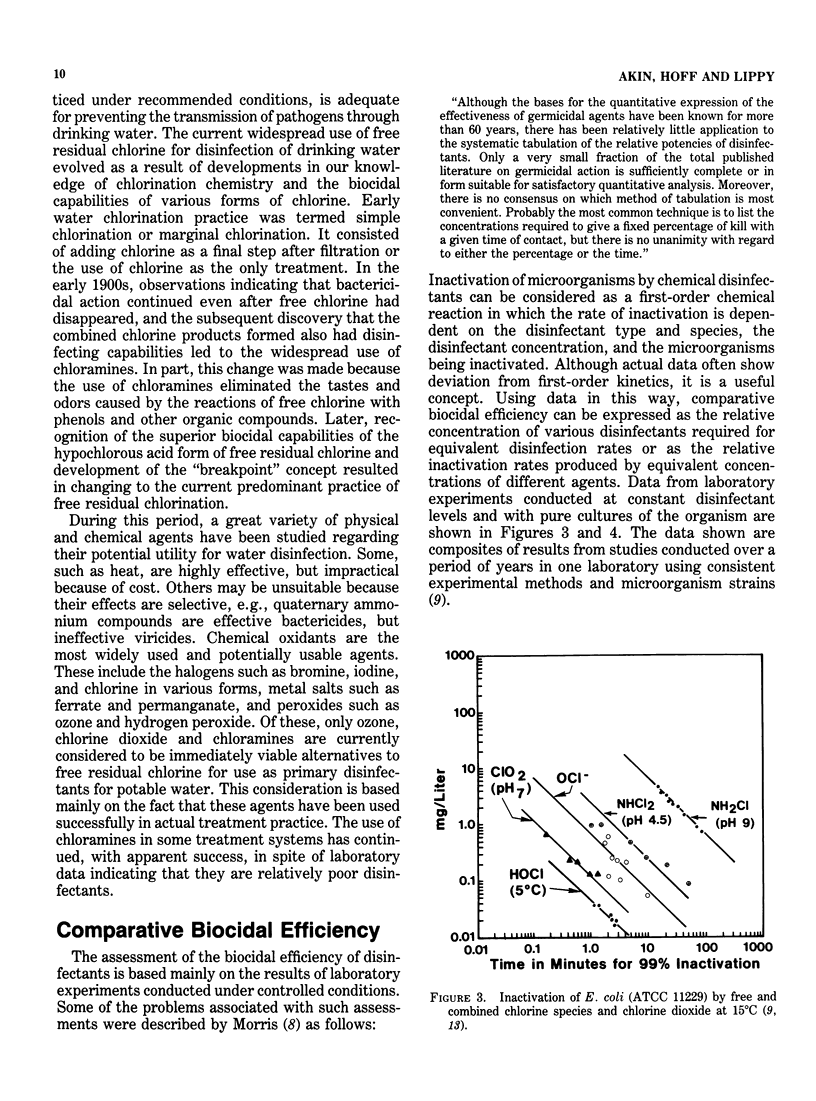
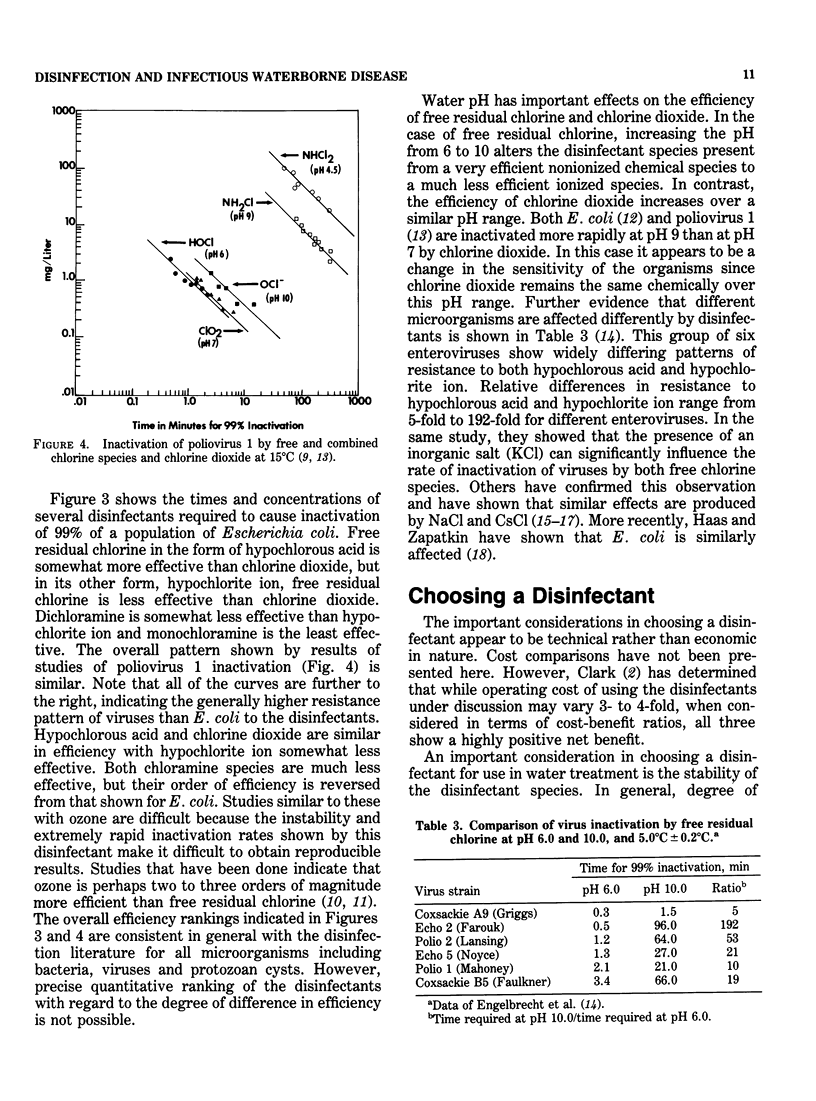
Drinking water disinfection was shown to be an important public health measure around the turn of the century. In the United States, it was perhaps the single most important factor in controlling typhoid fever, a waterborne disease that was rampant throughout the world during the last century. It may also be assumed that disinfection was important in limiting the number of cases of other diseases known to be capable of waterborne transmission, i.e., cholera, amebiasis, shigellosis, salmonellosis, and hepatitis A. Even though modern treatment has eliminated water as a major vehicle of infectious disease transmission, outbreaks still occur. In fact, the annual number has been increasing since 1966. Interruption in chlorination or failure to achieve adequate levels of chlorine residual is the most often identified deficiency of the involved water supplies. This finding indicates that waterborne microbial pathogens remain as a potential health threat and underscores the importance of disinfection. From the outset, chlorination has been the drinking water disinfectant of choice in the country. Numerous studies have demonstrated its ability to inactivate bacterial, viral, and protozoal pathogens when applied under proper conditions. However, the finding that chlorinated organics that are potentially carcinogenic are formed has prompted an evaluation of alternative disinfectants. The viable alternatives to chlorine currently under consideration for widespread use are ozone, chlorine dioxide, and chloramines. In terms of biocidal efficiency, ozone is the most potent of the three. Chlorine dioxide is about the equivalent of free chlorine in the hypochlorous acid form but much more efficient than the hypochlorite form of free chlorine. The chloramines are weaker biocides than hypochlorite. Although this general order of ranking of efficiency holds for diverse types of microorganisms, quantitative comparisons vary with different microorganisms and experimental conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benarde M. A., Israel B. M., Olivieri V. P., Granstrom M. L. Efficiency of chlorine dioxide as a bactericide. Appl Microbiol. 1965 Sep;13(5):776–780. doi: 10.1128/am.13.5.776-780.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht R. S., Weber M. J., Salter B. L., Schmidt C. A. Comparative inactivation of viruses by chlorine. Appl Environ Microbiol. 1980 Aug;40(2):249–256. doi: 10.1128/aem.40.2.249-256.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarroll E. L., Bingham A. K., Meyer E. A. Effect of chlorine on Giardia lamblia cyst viability. Appl Environ Microbiol. 1981 Feb;41(2):483–487. doi: 10.1128/aem.41.2.483-487.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen H., Thomas K., Sharp D. G. Inactivation of coxsackieviruses B3 and B5 in water by chlorine. Appl Environ Microbiol. 1980 Sep;40(3):633–640. doi: 10.1128/aem.40.3.633-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Leong J. Inactivation of poliovirus I (Brunhilde) single particles by chlorine in water. Appl Environ Microbiol. 1980 Aug;40(2):381–385. doi: 10.1128/aem.40.2.381-385.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Young D. C., Floyd R., Johnson J. D. Effect of ionic environment on the inactivation of poliovirus in water by chlorine. Appl Environ Microbiol. 1980 Mar;39(3):530–534. doi: 10.1128/aem.39.3.530-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


