Abstract
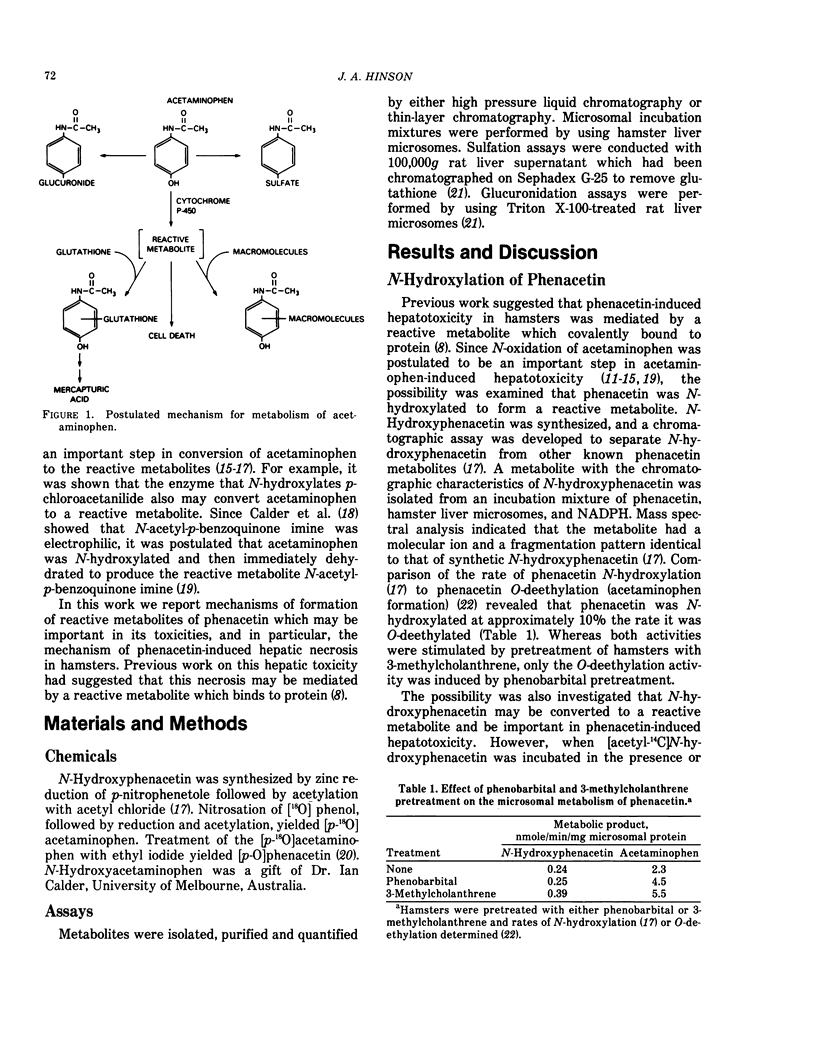
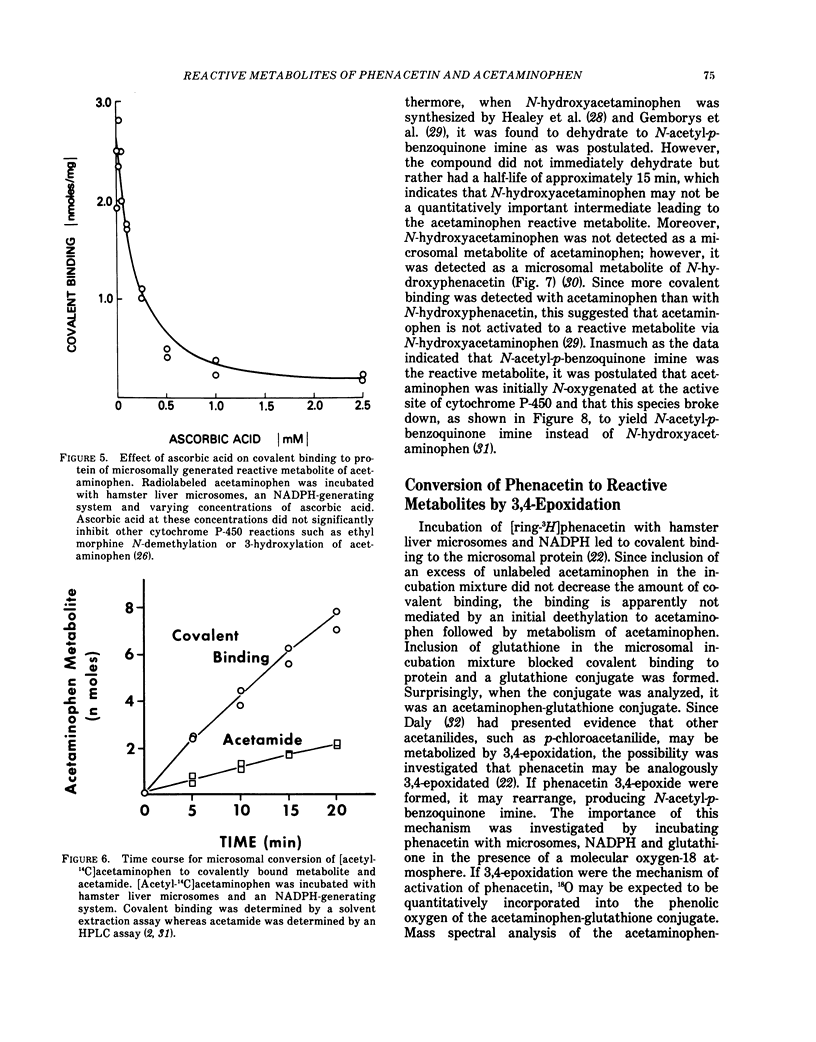
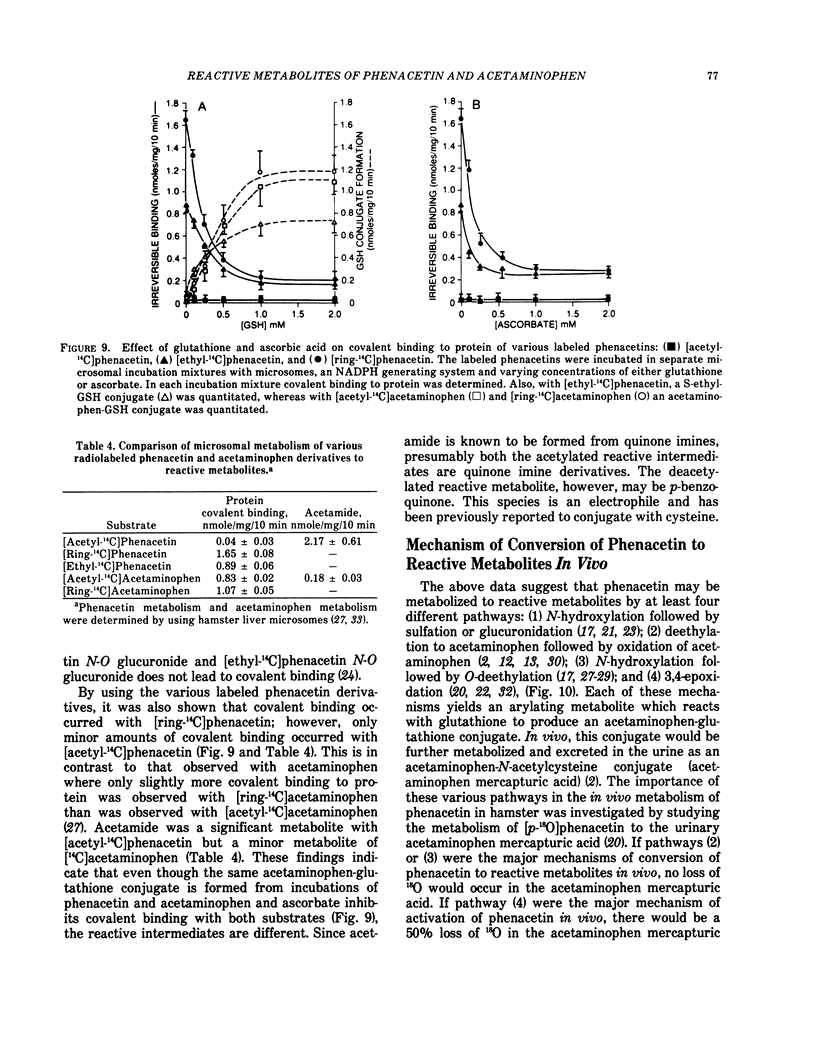
Phenacetin can be metabolized to reactive metabolites by a variety of mechanisms. (1) Phenacetin can be N-hydroxylated, and the resulting N-hydroxyphenacetin can be sulfated or glucuronidated. Whereas phenacetin N-O sulfate immediately rearranges to form a reactive metabolite which may covalently bind to protein, phenacetin N-O glucuronide slowly rearranges to form reactive metabolites. Incubation of the purified phenacetin N-O glucuronide under a variety of conditions suggests that N-acetyl-p-benzoquinone imine is a reactive metabolite. This metabolite covalently binds to protein, reacts with glutathione to form an acetaminophen-glutathione conjugate, is reduced by ascorbate to acetaminophen or is partially hydrolyzed to acetamide. (2) Phenacetin can be O-deethylated to acetaminophen, and acetaminophen can be converted directly to a reactive metabolite which may be also N-acetyl-p-benzoquinone imine. (3) Phenacetin can be sequentially N-hydroxylated and O-deethylated to N-hydroxyacetaminophen which spontaneously dehydrates to N-acetyl-p-benzoquinone imine. (4) Phenacetin can be 3, 4-epoxidated to form an alkylating and an arylating metabolite. In the presence of glutathione, a S-ethylglutathione conjugate and an acetaminophen-glutathione conjugate are formed. In the absence of glutathione, the alkylating metabolite may bind to protein and the arylating metabolite is completely hydrolyzed to acetamide and another arylating metabolite which may bind to protein. The structures of the alkylating and arylating metabolites are unknown.
Control experiments have shown that in pathway (1) the phenolic oxygen of the acetaminophenglutathione conjugate is derived from water, whereas in pathways (2) and (3) the phenolic oxygen of this metabolite is derived from phenacetin. In pathway (4) the phenolic oxygen was 50% derived from molecular oxygen and 50% from phenacetin. Administration of [p-180]phenacetin to hamsters revealed only a 10% loss of 180 in the acetaminophen mercapturic acid (the further metabolic product of the glutathione conjugate) which suggests that, in the hamster, pathways (2) and/or (3) are the primary mechanism of conversion of phenacetin to reactive metabolites in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengtsson U., Johansson S., Angervall L. Malignancies of the urinary tract and their relation to analgesic abuse. Kidney Int. 1978 Jan;13(1):107–113. doi: 10.1038/ki.1978.13. [DOI] [PubMed] [Google Scholar]
- Boyd E. M., Bereczky G. M. Liver necrosis from paracetamol. Br J Pharmacol Chemother. 1966 Mar;26(3):606–614. doi: 10.1111/j.1476-5381.1966.tb01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder I. C., Creek M. J., Williams P. J., Funder C. C., Green C. R., Ham K. N., Tange J. D. N-hydroxylation of p-acetophenetidide as a factor in nephrotoxicity. J Med Chem. 1973 May;16(5):499–502. doi: 10.1021/jm00263a019. [DOI] [PubMed] [Google Scholar]
- Daly J. Metabolism of acetanilides and anisoles with rat liver microsomes. Biochem Pharmacol. 1970 Dec;19(12):2979–2993. doi: 10.1016/0006-2952(70)90084-5. [DOI] [PubMed] [Google Scholar]
- Davidson D. G., Eastham W. N. Acute liver necrosis following overdose of paracetamol. Br Med J. 1966 Aug 27;2(5512):497–499. doi: 10.1136/bmj.2.5512.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaun J. R., Miller E. C., Miller J. A. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970 Mar;30(3):577–595. [PubMed] [Google Scholar]
- Gemborys M. W., Gribble G. W., Mudge G. H. Synthesis of N-hydroxyacetaminophen, a postulated toxic metabolite of acetaminophen, and its phenolic sulfate conjugate. J Med Chem. 1978 Jul;21(7):649–652. doi: 10.1021/jm00205a011. [DOI] [PubMed] [Google Scholar]
- Healey K., Calder I. C., Yong A. C., Crowe C. A., Funder C. C., Ham K. N., Tange J. D. Liver and kidney damage induced by N-hydroxyparacetamol. Xenobiotica. 1978 Jul;8(7):403–411. doi: 10.3109/00498257809070024. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Andrews L. S., Gillette J. R. Kinetic evidence for multiple chemically reactive intermediates in the breakdown of phenacetin N-O-glucuronide. Pharmacology. 1979;19(5):237–248. doi: 10.1159/000137318. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Mitchell J. R., Jollow D. J. N-hydroxylation of p-chloroacetanilde in hamsters. Biochem Pharmacol. 1976 Mar 1;25(5):599–601. doi: 10.1016/0006-2952(76)90394-4. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Mitchell J. R. N-Hydroxylation of phenacetin by hamster liver microsomes. Drug Metab Dispos. 1976 Sep-Oct;4(5):430–435. [PubMed] [Google Scholar]
- Hinson J. A., Nelson S. D., Gillette J. R. Metabolism of [p-18O]-phenacetin: the mechanism of activation of phenacetin to reactive metabolites in hamsters. Mol Pharmacol. 1979 Mar;15(2):419–427. [PubMed] [Google Scholar]
- Hinson J. A., Nelson S. D., Mitchell J. R. Studies on the microsomal formation of arylating metabolites of acetaminophen and phenacetin. Mol Pharmacol. 1977 Jul;13(4):625–633. [PubMed] [Google Scholar]
- Hinson J. A., Pohl L. R., Gillette J. R. N-Hydroxyacetaminophen: a microsomal metabolite of N-hydroxyphenacetin but apparently not of acetaminophen. Life Sci. 1979 Jun 4;24(23):2133–2138. doi: 10.1016/0024-3205(79)90111-5. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Pohl L. R., Monks T. J., Gillette J. R. Acetaminophen-induced hepatotoxicity. Life Sci. 1981 Jul 13;29(2):107–116. doi: 10.1016/0024-3205(81)90278-2. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Pohl L. R., Monks T. J., Gillette J. R., Guengerich F. P. 3-Hydroxyacetaminophen: a microsomal metabolite of acetaminophen. Evidence against an epoxide as the reactive metabolite of acetaminophen. Drug Metab Dispos. 1980 Sep-Oct;8(5):289–294. [PubMed] [Google Scholar]
- Hultengren N., Lagergren C., Ljungqvist A. Carcinoma of the renal pelvis in renal papillary necrosis. Acta Chir Scand. 1965 Oct;130(4):314–320. [PubMed] [Google Scholar]
- Isaka H., Yoshii H., Otsuji A., Koike M., Nagai Y., Koura M., Sugiyasu K., Kanabayashi T. Tumors of Sprague-Dawley rats induced by long-term feeding of phenacetin. Gan. 1979 Feb;70(1):29–36. [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973 Oct;187(1):195–202. [PubMed] [Google Scholar]
- Jollow D. J., Thorgeirsson S. S., Potter W. Z., Hashimoto M., Mitchell J. R. Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology. 1974;12(4-5):251–271. doi: 10.1159/000136547. [DOI] [PubMed] [Google Scholar]
- Linton A. L. Renal disease due to analgesics. I. Recognition of the problem of analgesic nephropathy. Can Med Assoc J. 1972 Oct 21;107(8):749–751. [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973 Oct;187(1):185–194. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- Mulder G. J., Hinson J. A., Gillette J. R. Conversion of the N-Q-glucuronide and N-O-sulfate conjugates of N-hydroxyphenacetin to reactive intermediates. Biochem Pharmacol. 1978;27(12):1641–1649. doi: 10.1016/0006-2952(78)90173-9. [DOI] [PubMed] [Google Scholar]
- Mulder G. J., Hinson J. A., Gillette J. R. Generation of reactive metabolites of N-hydroxy-phenacetin by glucoronidation and sulfation. Biochem Pharmacol. 1977 Feb 1;26(3):189–196. doi: 10.1016/0006-2952(77)90301-x. [DOI] [PubMed] [Google Scholar]
- Murray T., Goldberg M. Analgesic abuse and renal disease. Annu Rev Med. 1975;26:537–550. doi: 10.1146/annurev.me.26.020175.002541. [DOI] [PubMed] [Google Scholar]
- Nelson S. D., Forte A. J., Vaishnav Y., Mitchell J. R., Gillette J. R., Hinson J. A. The formation of arylating and alkylating metabolites of phenacetin in hamsters and hamster liver microsomes. Mol Pharmacol. 1981 Jan;19(1):140–145. [PubMed] [Google Scholar]
- Potter W. Z., Davis D. C., Mitchell J. R., Jollow D. J., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther. 1973 Oct;187(1):203–210. [PubMed] [Google Scholar]


