Abstract
N-Hydroxylation and mutagenic activation of heterocyclic aromatic amines from protein pyrolysis products were studied in rat liver microsomes and nuclei, rat hepatocytes and various species of purified cytochrome P-450. These mutagenic amines include Trp-P-2 (3-amino-1-methyl-5H-pyrido[4,3-b]indole), Trp-P-1 (3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole), Glu-P-1 (2-amino-6-methyldipyrido-[1,2-a:3',2'-d]imidazole), Glu-P-2 (2-amino-dipyrido[1,2-a:3',2'-d]imidazole) and IQ (2-amino-3-methyl-3H-imidazo-[4,5-f]quinoline). The number of revertants of Salmonella typhimurium TA 98 was always correlated to the amount of each of the N-hydroxylated metabolites in various experimental conditions. The N-hydroxylated amines covalently bound to DNA directly or after being acylated with amino acids by amino-acyl-tRNA synthetase. Various species of cytochrome P-450 preparations showed markedly different activity in N-hydroxylation and mutagenic activation of Trp-P-2, Glu-P-1 and IQ. A high spin form of cytochrome P-450, isolated from the liver of PCB-treated rats, showed very high activity in N-hydroxylation of Trp-P-2, Glu-P-1 and 2-aminofluorene, although its activity was very low in benzo(a)pyrene hydroxylation. The present results indicate that different species of cytochrome P-450 are involved in the N-hydroxylation and mutagenic activation of aromatic amines.
Full text
PDF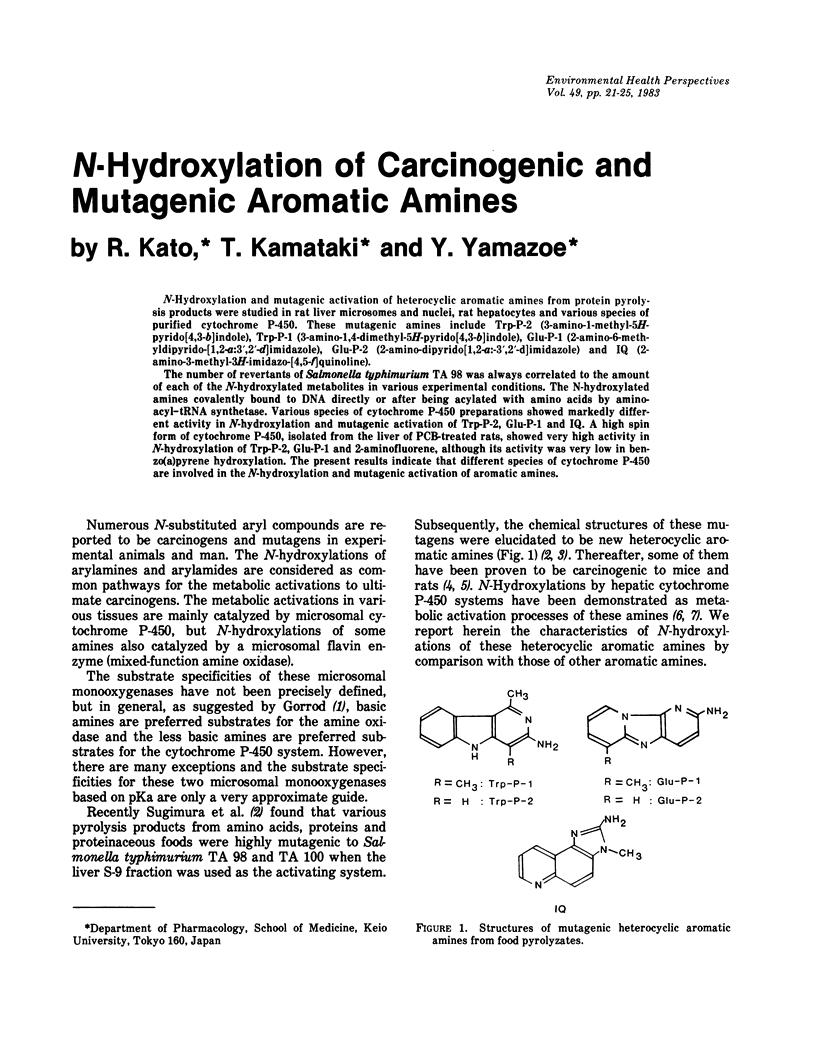


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gorrod J. W. Differentiation of various types of biological oxidation of nitrogen in organic compounds. Chem Biol Interact. 1973 Nov;289(303):289–303. doi: 10.1016/0009-2797(73)90004-5. [DOI] [PubMed] [Google Scholar]
- Ishii K., Ando M., Kamataki T., Kato R., Nagao M. Metabolic activation of mutagenic tryptophan pyrolysis products (Trp-P-1 and Trp-P-2) by a purified cytochrome P-450-dependent monooxygenase system. Cancer Lett. 1980 Jun;9(4):271–276. doi: 10.1016/0304-3835(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Ishii K., Yamazoe Y., Kamataki T., Kato R. Metabolic activation of mutagenic tryptophan pyrolysis products by rat liver microsomes. Cancer Res. 1980 Jul;40(7):2596–2600. [PubMed] [Google Scholar]
- Kadlubar F. F., Miller J. A., Miller E. C. Guanyl O6-arylamination and O6-arylation of DNA by the carcinogen N-hydroxy-1-naphthylamine. Cancer Res. 1978 Nov;38(11 Pt 1):3628–3638. [PubMed] [Google Scholar]
- Matsukura N., Kawachi T., Morino K., Ohgaki H., Sugimura T., Takayama S. Carcinogenicity in mice of mutagenic compounds from a tryptophan pyrolyzate. Science. 1981 Jul 17;213(4505):346–347. doi: 10.1126/science.7244619. [DOI] [PubMed] [Google Scholar]
- Mita S., Ishii K., Yamazoe Y., Kamataki T., Kato R., Sugimura T. Evidence for the involvement of N-hydroxylation of 3-amino-1-methyl-5H-pyrido[4,3-b]indole by cytochrome P-450 in the covalent binding to DNA. Cancer Res. 1981 Sep;41(9 Pt 1):3610–3614. [PubMed] [Google Scholar]
- Mita S., Yamazoe Y., Kamataki T., Kato R. Effects of ascorbic acid on the nonenzymatic binding to DNA and the mutagenicity of N-hydroxylated metabolite of a tryptophan-pyrolysis product. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1396–1401. doi: 10.1016/0006-291x(82)90942-1. [DOI] [PubMed] [Google Scholar]
- Nagao M., Sugimura T., Matsushima T. Environmental mutagens and carcinogens. Annu Rev Genet. 1978;12:117–159. doi: 10.1146/annurev.ge.12.120178.001001. [DOI] [PubMed] [Google Scholar]
- Razzouk C., Mercier M., Roberfroid M. Characterization of the guinea pig liver microsomal 2-fluorenylamine and N-2-fluorenylacetamide N-hydroxylase. Cancer Lett. 1980 Apr;9(2):123–131. doi: 10.1016/0304-3835(80)90116-0. [DOI] [PubMed] [Google Scholar]
- Yamazoe Y., Ishii K., Kamataki T., Kato R., Sugimura T. Isolation and characterization of active metabolites of tryptophan-pyrolysate mutagen, TRP-P-2, formed by rat liver microsomes. Chem Biol Interact. 1980 May;30(2):125–138. doi: 10.1016/0009-2797(80)90120-9. [DOI] [PubMed] [Google Scholar]
- Yamazoe Y., Kamataki T., Kato R. Species difference in N-hydroxylation of a tryptophan pyrolysis product in relation to mutagenic activation. Cancer Res. 1981 Nov;41(11 Pt 1):4518–4522. [PubMed] [Google Scholar]
- Yamazoe Y., Tada M., Kamataki T., Kato R. Enhancement of Binding of N-Hydroxy-TRP-P-2 to DNA by seryl-tRNA synthetase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):432–439. doi: 10.1016/0006-291x(81)91539-4. [DOI] [PubMed] [Google Scholar]


