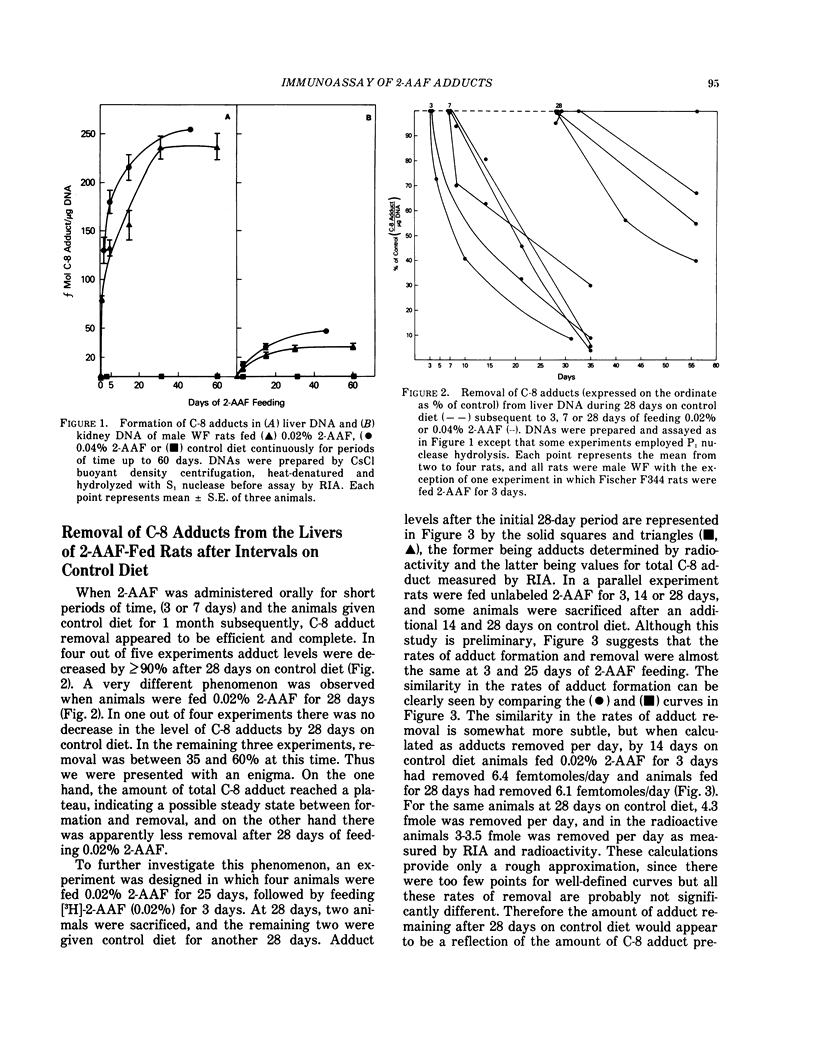
Abstract
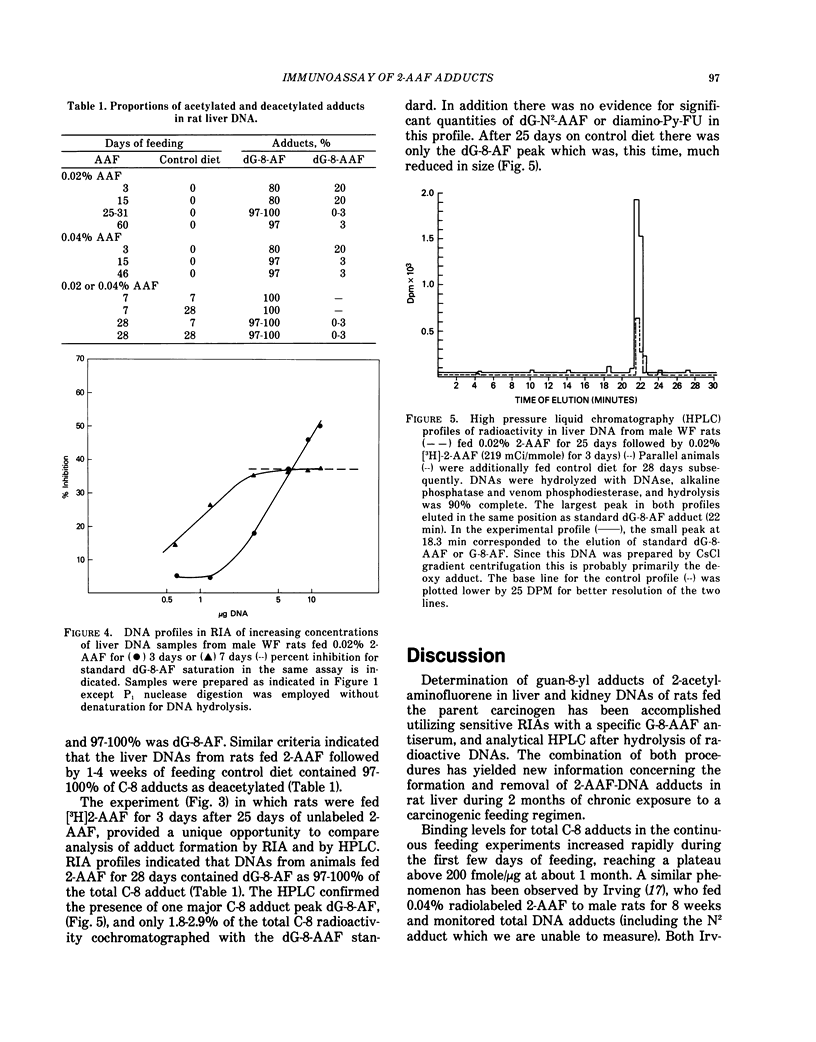
Antisera elicited in rabbits were used in radioimmunoassay (RIA) and enzyme-linked immunosorbent assay (ELISA) to determine femtomole quantities of deoxyguanosin-(8-yl)-acetylaminofluorene (dg-8-AAF) and deoxyguanosin-(8-yl)-aminofluorene (dg-8-AF). These adducts have been monitored in liver and kidney DNA of male Wistar-Furth rats fed 0.02% or 0.04% 2-acetylaminofluorene (2-AAF) either continuously or for a limited time followed by an interval on control diet. After 24 hr of 0.02% 2-AAF feeding, substantial levels of binding (80 fmole/μg DNA) were observed in liver DNA and increased with time, reaching a plateau of approximately 230 fmole/μg DNA at 30 days and thereafter. During the first week of continuous feeding about 80% of the total C-8 adducts in the liver DNA were deacetylated (dG-8-AF). By 25-60 days, dG-8-AF represented 97-100% of all C-8 adducts as measured by RIA and confirmed by HPLC. Values for C-8 adduct formation in kidney DNA were severalfold lower than in liver and dG-8-AF represented >90% of C-8 adducts at all times studied.
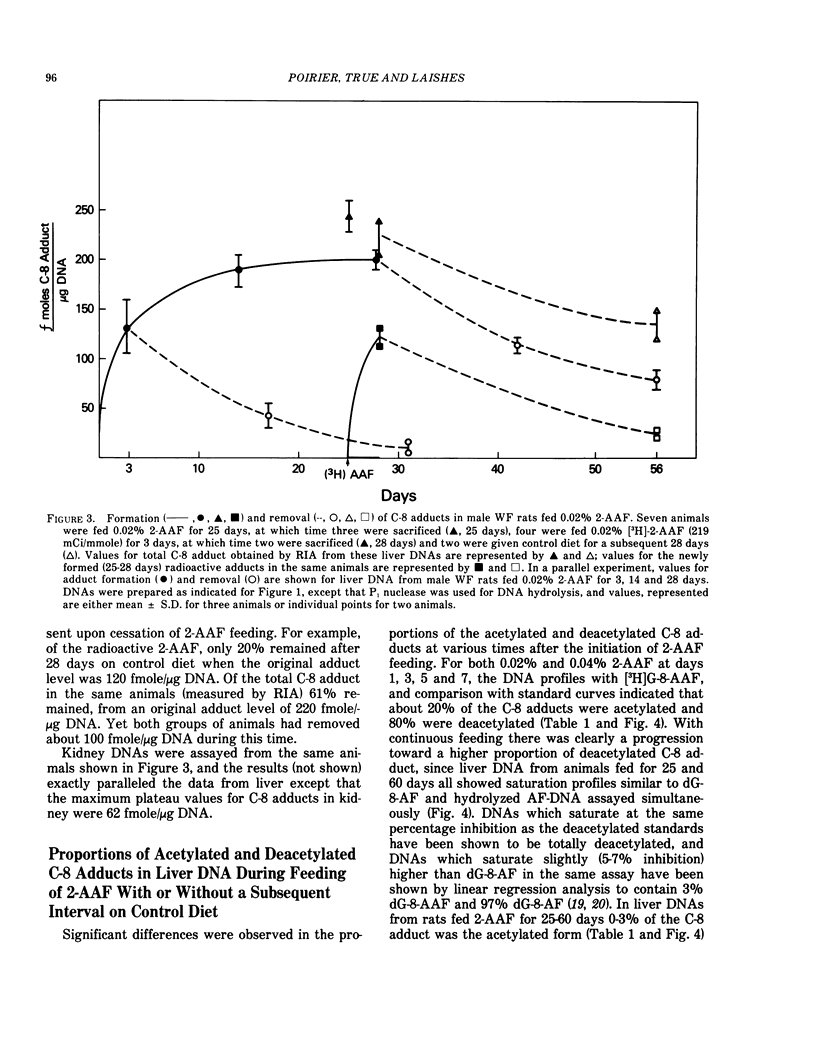
In removal or repair experiments, rats were fed 2-AAF for 3, 7 or 28 days, the 2-AAF diet was discontinued and the liver adducts assayed after intervals on control diet. When dietary 2-AAF administration was for 3 or 7 days, removal of adducts was efficient and almost complete by 28 days on control diet, with preferential retention of dG-8-AF. However, when dietary 2-AAF administration was for 28 days, adduct levels were higher, the repair capacity was saturated and the removal of C-8 adducts was not complete after control diet for a 28-day interval. In a preliminary experiment when [3H]-2-AAF was fed for 3 days, after 25 days of 0.02% 2-AAF, the rates of newly formed adduct formation and removal were similar to those observed for the initial 3 days of 2-AAF feeding. These results demonstrate the predominance and persistence of dG-8-AF in liver and kidney DNA of 2-AAF-fed rats and suggest that the repair capacity of the whole rat liver was not diminished after 1 month of 2-AAF feeding.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beland F. A., Dooley K. L., Casciano D. A. Rapid isolation of carcinogen-bound DNA and RNA by hydroxyapatite chromatography. J Chromatogr. 1979 Jun 1;174(1):177–186. doi: 10.1016/s0021-9673(00)87048-x. [DOI] [PubMed] [Google Scholar]
- Howard P. C., Casciano D. A., Beland F. A., Shaddock J. G., Jr The binding of N-hydroxy-2-acetylaminofluorene to DNA and repair of the adducts in primary rat hepatocyte cultures. Carcinogenesis. 1981;2(2):97–102. doi: 10.1093/carcin/2.2.97. [DOI] [PubMed] [Google Scholar]
- Irving C. C. Metabolic activation of N-hydroxy compounds by conjugation. Xenobiotica. 1971 Jul-Oct;1(4):387–398. doi: 10.3109/00498257109041505. [DOI] [PubMed] [Google Scholar]
- Irving C. C., Veazey R. A. Persistent binding of 2-acetylaminofluorene to rat liver DNA in vivo and consideration of the mechanism of binding of N-hydroxy-2-acetylaminofluorene to rat liver nucleic acids. Cancer Res. 1969 Oct;29(10):1799–1804. [PubMed] [Google Scholar]
- Kriek E. Carcinogenesis by aromatic amines. Biochim Biophys Acta. 1974 Sep 9;355(2):177–203. doi: 10.1016/0304-419x(74)90003-1. [DOI] [PubMed] [Google Scholar]
- Kriek E. Persistent binding of a new reaction product of the carcinogen N-hydroxy-N-2-acetylaminofluorene with guanine in rat liver DNA in vivo. Cancer Res. 1972 Oct;32(10):2042–2048. [PubMed] [Google Scholar]
- Laishes B. A., Rolfe P. B. Quantitative assessment of liver colony formation and hepatocellular carcinoma incidence in rats receiving intravenous injections of isogeneic liver cells isolated during hepatocarcinogenesis. Cancer Res. 1980 Nov;40(11):4133–4143. [PubMed] [Google Scholar]
- Lieberman M. W., Poirier M. C. Deoxyribonucleoside incorporation during DNA repair of carcinogen-induced damage in human diploid fibroblasts. Cancer Res. 1973 Sep;33(9):2097–2103. [PubMed] [Google Scholar]
- Poirier M. C. Antibodies to carcinogen-DNA adducts. J Natl Cancer Inst. 1981 Sep;67(3):515–519. [PubMed] [Google Scholar]
- Poirier M. C., Connor R. J. Radioimmunoassay for 2-acetylaminofluorene-DNA adducts. Methods Enzymol. 1982;84:607–618. doi: 10.1016/0076-6879(82)84048-2. [DOI] [PubMed] [Google Scholar]
- Poirier M. C., Dubin M. A., Yuspa S. H. Formation and removal of specific acetylaminofluorene-DNA adducts in mouse and human cells measured by radioimmunoassay. Cancer Res. 1979 Apr;39(4):1377–1381. [PubMed] [Google Scholar]
- Poirier M. C., Yuspa S. H., Weinstein I. B., Blobstein S. Detection of carcinogen-DNA adducts by radiommunoassay. Nature. 1977 Nov 10;270(5633):186–188. doi: 10.1038/270186a0. [DOI] [PubMed] [Google Scholar]
- Szafarz D., Weisburger J. H. Stability of binding of label from N-hydroxy-N-2-fluorenylacetamide to intracellular targets, particularly deoxyribonucleic acid in rat liver. Cancer Res. 1969 Apr;29(4):962–968. [PubMed] [Google Scholar]
- Visser A., Westra J. G. Partial persistency of 2-aminofluorene and N-acetyl-2-aminofluorene in rat liver DNA. Carcinogenesis. 1981;2(8):737–740. doi: 10.1093/carcin/2.8.737. [DOI] [PubMed] [Google Scholar]
- Witschi H., Epstein S. M., Farber E. Influence of liver regeneration on the loss of fluorenylacetamide derivative bound to liver DNA. Cancer Res. 1971 Mar;31(3):270–273. [PubMed] [Google Scholar]


