Abstract
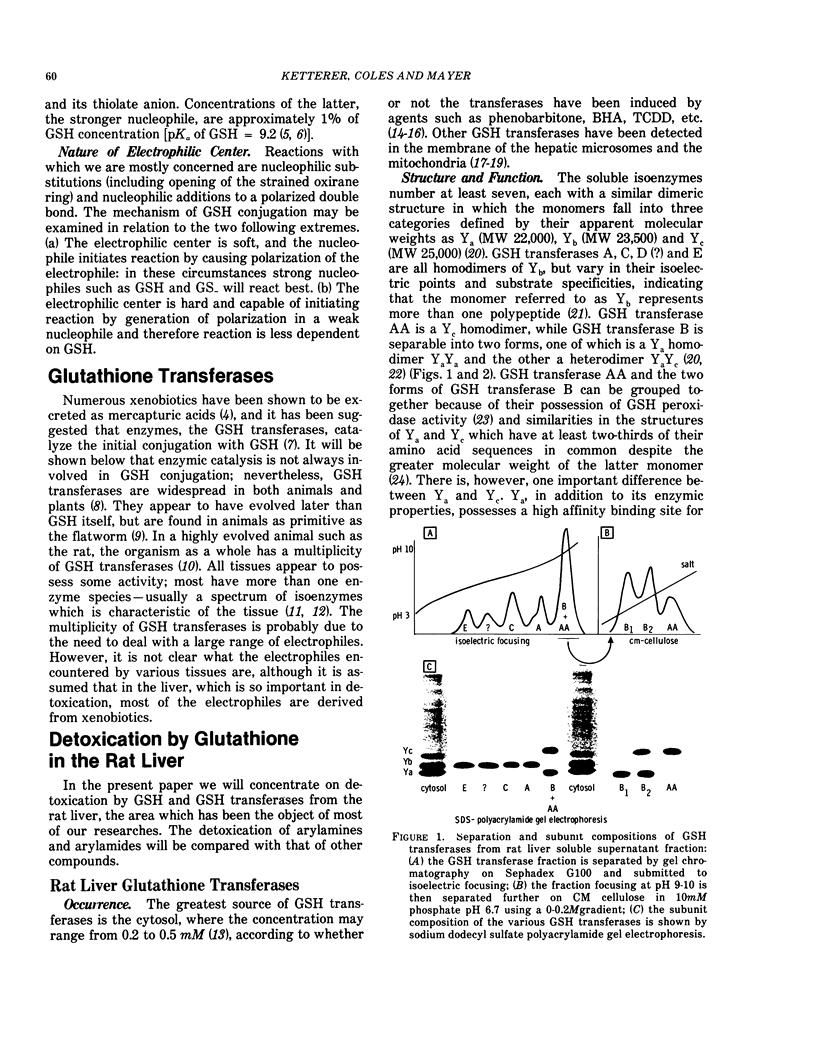
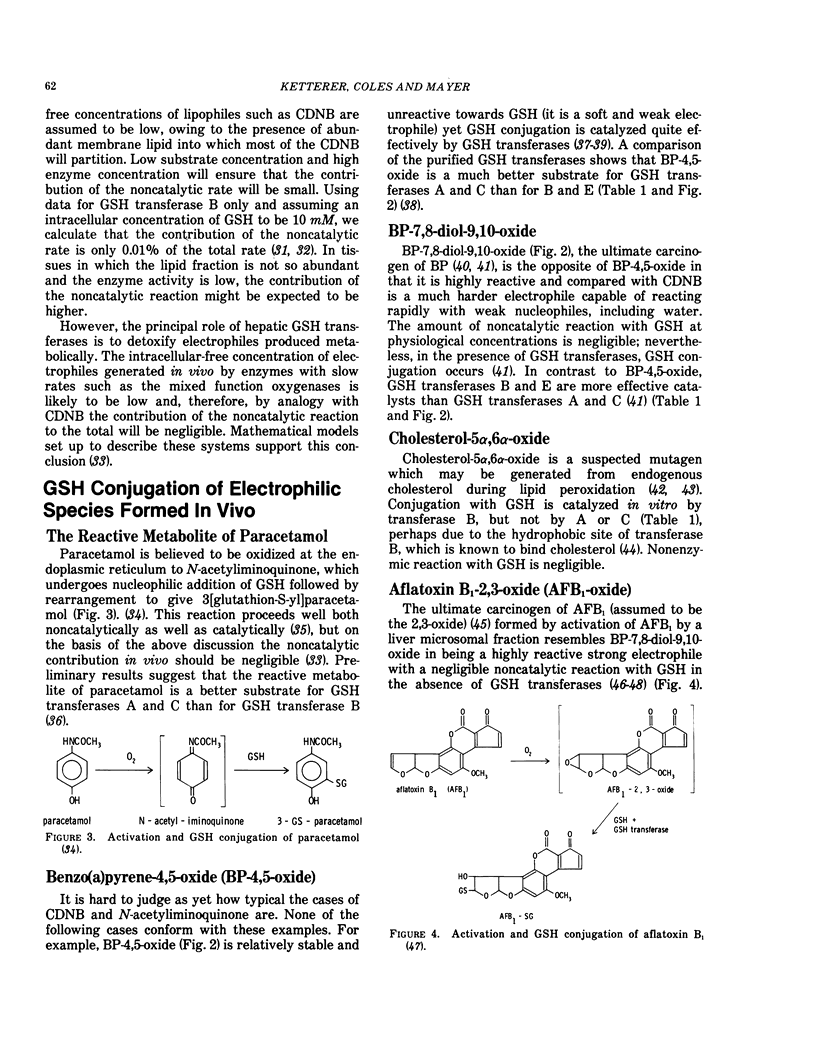
Glutathione (GSH) is a strong nucleophile which reacts well with soft electrophiles, but poorly with both weak and strong electrophiles. Weak electrophiles have low reactivity with all nucleophiles while strong electrophiles react well with weak nucleophiles including superabundant H2O.
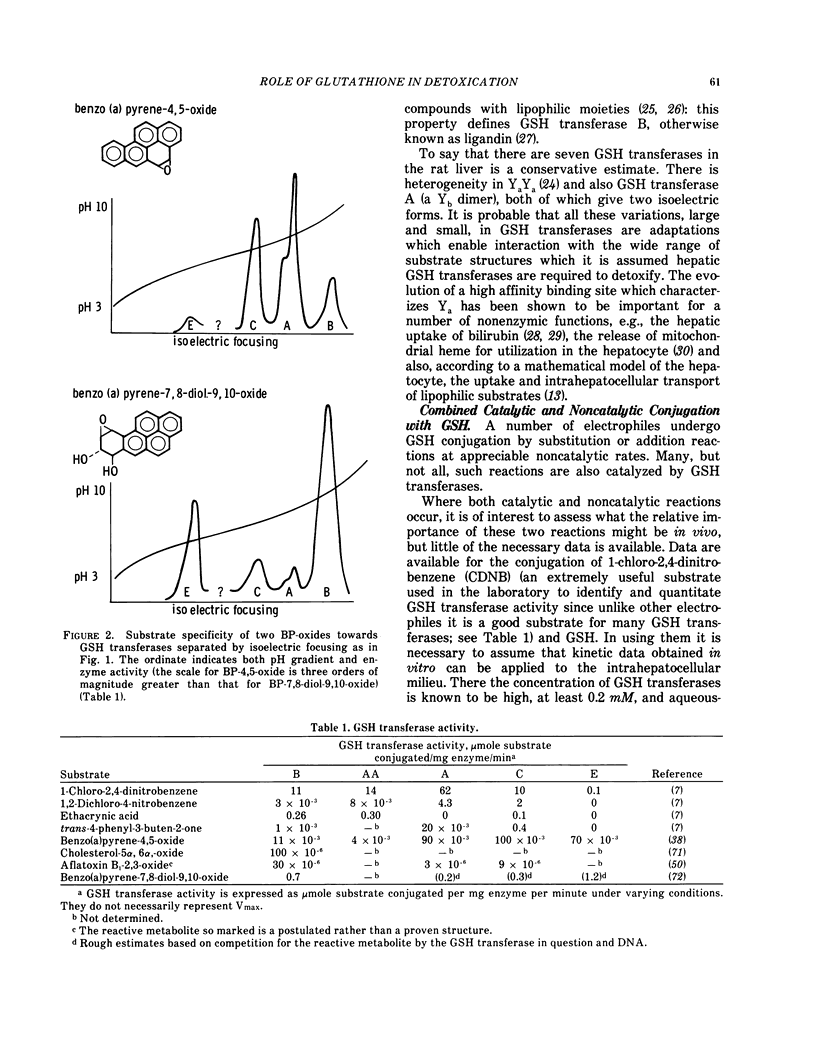
There are enzymes, the GSH transferases, which catalyze GSH conjugation with all the types of electrophiles described above. In order to deal with the wide variety of potential substrates, a multiplicity of GSH transferases exists—each tissue having its own collection and each enzyme having a different substrate specificity. These enzymes are often very abundant, e.g., in the rat liver cytosol, their concentration is 0.2 mM.
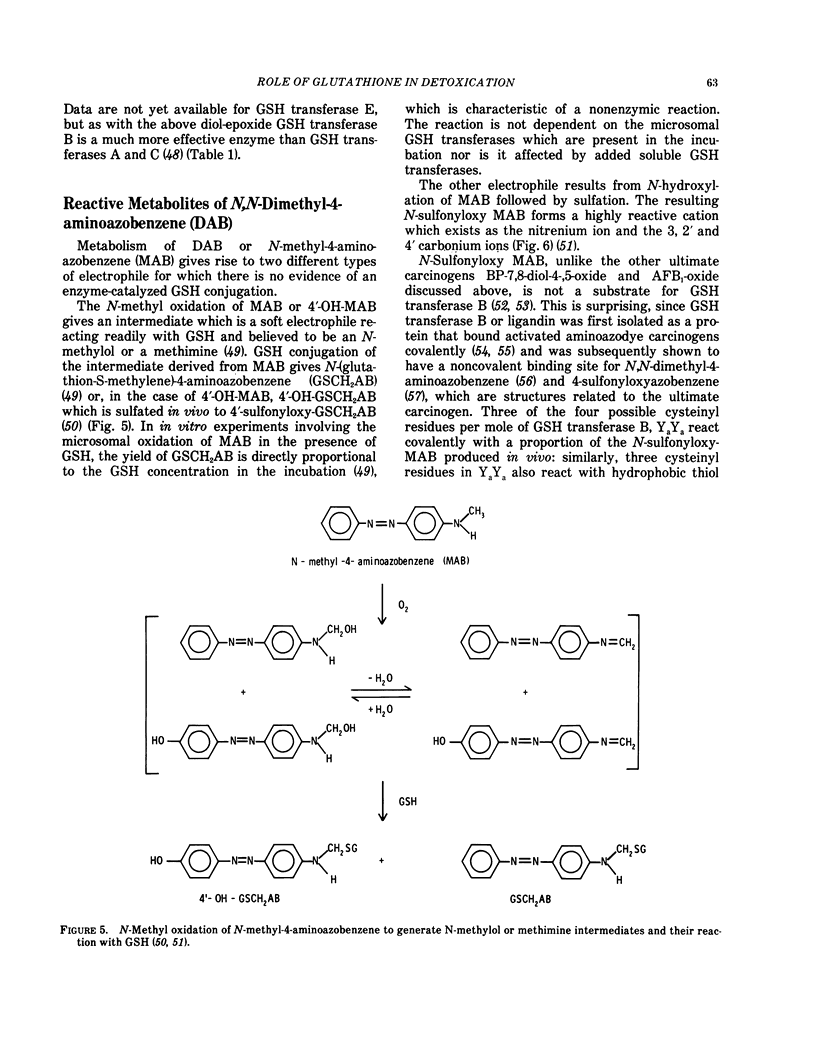
The following substrates are considered in some detail: 1-chloro-2,4-dinitrobenzene, the electrophile derived metabolically from paracetamol N-acetyliminoquinone?), benzo(a)pyrene-4-5-oxide, cholesterol-5α,6α-oxide, benzo(a)pyrene-7,8-diol-9,10-oxide and the electrophiles derived metabolically from aflatoxin B1 (the 2,3-oxide?). According to the substrate, optimal enzyme rates vary over seven orders of magnitude from 10−5 to 10−12 mole/min/mg.
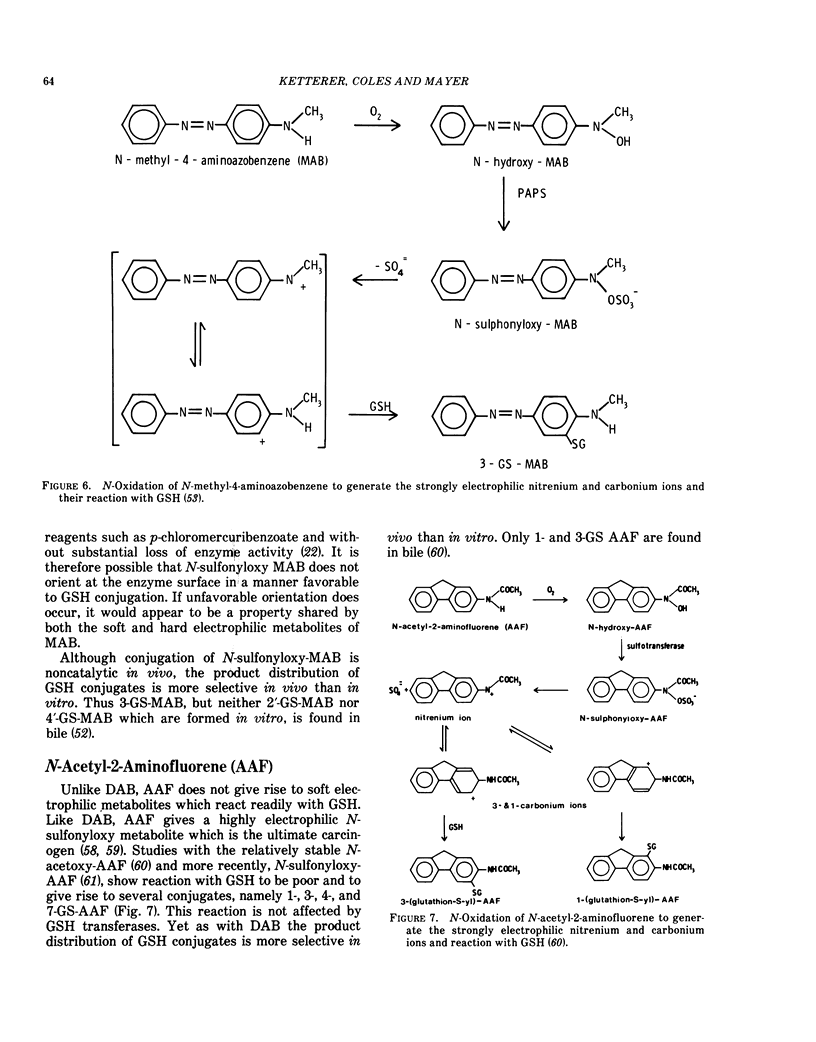
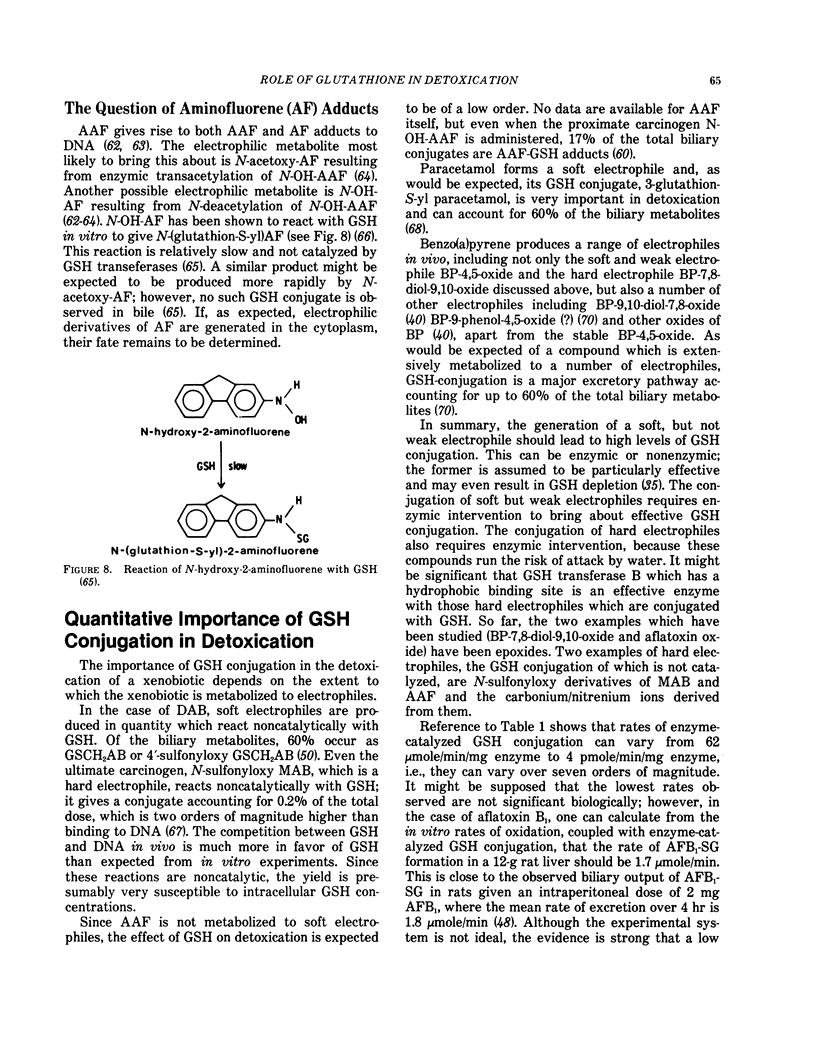
Despite the wide embrace of the GSH transferases, not all metabolically produced electrophiles are substrates. We know of the following examples: N-methylol-4-aminoazobenzene and its 4′-hydroxy derivative (these are soft electrophiles and react well with GSH noncatalytically), N-sulfonyloxy-N-methyl-4-aminoazobenzene, N-sulfonyloxy-N-acetyl-2-aminofluorene (these are strong electrophiles which do not react selectively with GSH) and N-hydroxy-2-aminofluorene which appears to react only slowly with GSH. It is of interest in the present context that all these compounds are derived from either arylamine or arylamide carcinogens.
Whether the reaction be enzymic or nonenzymic, conjugation with GSH is a very important means of detoxication accounting in some cases for up to 60% of the biliary metabolites. As seen in the example of aflatoxin B1, very low enzymic rates observed in vitro are sufficient to account for apparently high rates of biliary excretion of GSH conjugates.
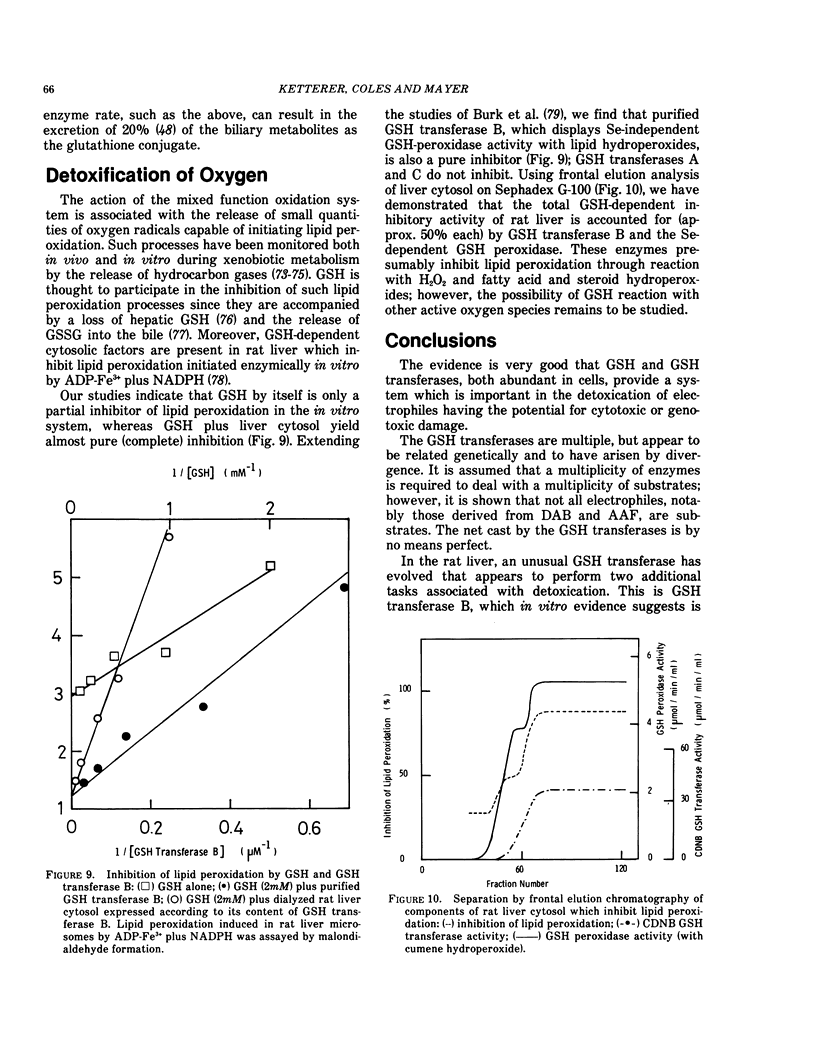
GSH transferases have evolved other functions apart from the catalysis of GSH conjugation. GSH transferase B participates in the hepatic uptake of bilirubin and the intracellular distribution of the heme prosthetic group. It also has GSH peroxidase activity which suggests that it might participate in the detoxication of by-products of oxygen utilization including those produced by the action of cytochrome P-450. It is shown that GSH transferase B inhibits lipid peroxidation in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bilzer M., Sies H. Competition between transport of glutathione disulfide (GSSG) and glutathione S-conjugates from perfused rat liver into bile. FEBS Lett. 1982 Apr 5;140(1):73–76. doi: 10.1016/0014-5793(82)80523-1. [DOI] [PubMed] [Google Scholar]
- Baars A. J., Jansen M., Breimer D. D. The influence of phenobarbital, 3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin on glutathione S-transferase activity of rat liver cytosol. Biochem Pharmacol. 1978;27(21):2487–2497. doi: 10.1016/0006-2952(78)90314-3. [DOI] [PubMed] [Google Scholar]
- Baars A. J., Mukhtar H., Zoetemelk C. E., Jansen M., Breimer D. D. Glutathione S-transferase activity in rat and human tissues and organs. Comp Biochem Physiol C. 1981;70(2):285–288. doi: 10.1016/0306-4492(81)90066-6. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Dworkin M., Miller J. A., Miller E. C. Electrophilic N-acetoxyaminoarenes derived from carcinogenic N-hydroxy-N-acetylaminoarenes by enzymatic deacetylation and transacetylation in liver. Biochim Biophys Acta. 1972 Dec 29;286(2):272–298. doi: 10.1016/0304-4165(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Bass N. M., Kirsch R. E., Tuff S. A., Marks I., Saunders S. J. Ligandin heterogeneity : evidence that the two non-identical subunits are the monomers of two distinct proteins. Biochim Biophys Acta. 1977 May 27;492(1):163–175. doi: 10.1016/0005-2795(77)90223-9. [DOI] [PubMed] [Google Scholar]
- Beale D., Ketterer B., Carne T., Meyer D., Taylor J. B. Evidence that the Ya and Yc subunits of glutathione transferase B (ligandin) are the products of separate genes. Eur J Biochem. 1982 Sep 1;126(3):459–463. doi: 10.1111/j.1432-1033.1982.tb06802.x. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Batzinger R. P., Ou S. Y., Bueding E., Cha Y. N., Talalay P. Elevation of hepatic glutathione S-transferase activities and protection against mutagenic metabolites of benzo(a)pyrene by dietary antioxidants. Cancer Res. 1978 Dec;38(12):4486–4495. [PubMed] [Google Scholar]
- Bhargava M. M., Ohmi N., Listowsky I., Arias I. M. Structural, catalytic, binding, and immunological properties associated with each of the two subunits of rat liver ligandin. J Biol Chem. 1980 Jan 25;255(2):718–723. [PubMed] [Google Scholar]
- Black H. S., Douglas D. R. Formation of a carcinogen of natural origin in the etiology of ultraviolet light-induced carcinogenesis. Cancer Res. 1973 Sep;33(9):2094–2096. [PubMed] [Google Scholar]
- Boroujerdi M., Kung H., Wilson A. G., Anderson M. W. Metabolism and DNA binding of benzo(a)pyrene in vivo in the rat. Cancer Res. 1981 Mar;41(3):951–957. [PubMed] [Google Scholar]
- Burk R. F., Trumble M. J., Lawrence R. A. Rat hepatic cytosolic glutathione-dependent enzyme protection against lipid peroxidation in the NADPH-microsomal lipid peroxidation system. Biochim Biophys Acta. 1980 Apr 18;618(1):35–41. doi: 10.1016/0005-2760(80)90051-x. [DOI] [PubMed] [Google Scholar]
- Carne T., Tipping E., Ketterer B. The binding and catalytic activities of forms of ligandin after modification of its thiol groups. Biochem J. 1979 Feb 1;177(2):433–439. doi: 10.1042/bj1770433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- DeBaun J. R., Miller E. C., Miller J. A. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970 Mar;30(3):577–595. [PubMed] [Google Scholar]
- Douch P. G., Buchanan L. L. Glutathione conjugation of some xenobiotics by Ascaris suum and Moniezia expansa. Xenobiotica. 1978 Mar;8(3):171–176. doi: 10.3109/00498257809060396. [DOI] [PubMed] [Google Scholar]
- Emerole G. O., Neskovic N., Dixon R. L. The detoxication of aflatoxin B1 with glutathione in the rat. Xenobiotica. 1979 Dec;9(12):737–743. doi: 10.3109/00498257909042342. [DOI] [PubMed] [Google Scholar]
- Friedberg T., Bentley P., Stasiecki P., Glatt H. R., Raphael D., Oesch F. The identification, solubilization, and characterization of microsome-associated glutathione S-transferases. J Biol Chem. 1979 Dec 10;254(23):12028–12033. [PubMed] [Google Scholar]
- Garner R. C., Miller E. C., Miller J. A. Liver microsomal metabolism of aflatoxin B 1 to a reactive derivative toxic to Salmonella typhimurium TA 1530. Cancer Res. 1972 Oct;32(10):2058–2066. [PubMed] [Google Scholar]
- Gee D. L., Tappel A. L. Production of volatile hydrocarbons by isolated hepatocytes: an in vitro model for lipid peroxidation studies. Toxicol Appl Pharmacol. 1981 Aug;60(1):112–120. doi: 10.1016/0041-008x(81)90141-1. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Gillette J. R. Kinetics of decomposition of chemically unstable metabolites in the presence of nucleophiles: derivation of equations used in graphical analyses. Pharmacology. 1980;20(2):64–86. doi: 10.1159/000137347. [DOI] [PubMed] [Google Scholar]
- Grafström R., Ormstad K., Moldéus P., Orrenius S. Paracetamol metabolism in the isolated perfused rat liver with further metabolism of a biliary paracetamol conjugate by the small intestine. Biochem Pharmacol. 1979 Dec 15;28(24):3573–3579. doi: 10.1016/0006-2952(79)90402-7. [DOI] [PubMed] [Google Scholar]
- Grover P. L., Sims P. Conjugations with glutathione. Distribution of glutathione S-aryltransferase in vertebrate species. Biochem J. 1964 Mar;90(3):603–606. doi: 10.1042/bj0900603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaa K. A., Sochor M., McLean P., Greenbaum A. L. Effect of quinolinic acid on the metabolite profile and regulation of carbohydrate and lipid metabolism in the liver of diabetic rats. Arch Biochem Biophys. 1981 Jan;206(1):1–7. doi: 10.1016/0003-9861(81)90058-8. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hernandez O., Walker M., Cox R. H., Foureman G. L., Smith B. R., Bend J. R. Regiospecificity and stereospecificity in the enzymatic conjugation of glutathione with (+/-)-benzo(a)pyrene 4,5-oxide. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1494–1502. doi: 10.1016/0006-291x(80)91343-1. [DOI] [PubMed] [Google Scholar]
- Hesse S., Jernström B., Martinez M., Moldéus P., Christodoulides L., Ketterer B. Inactivation of DNA-binding metabolites of benzo[a]pyrene and benzo[a]pyrene-7,8-dihydrodiol by glutathione and glutathione S-transferases. Carcinogenesis. 1982;3(7):757–761. doi: 10.1093/carcin/3.7.757. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Monks T. J., Hong M., Highet R. J., Pohl L. R. 3-(glutathion-S-yl)acetaminophen: a biliary metabolite of acetaminophen. Drug Metab Dispos. 1982 Jan-Feb;10(1):47–50. [PubMed] [Google Scholar]
- Husby P., Srai K. S., Ketterer B., Romslo I. Effect of ligandin on the efflux of Co-deuteroporphyrin from isolated rat liver mitochondria. Biochem Biophys Res Commun. 1981 May 29;100(2):651–659. doi: 10.1016/s0006-291x(81)80225-2. [DOI] [PubMed] [Google Scholar]
- Irving C. C., Veazey R. A. Persistent binding of 2-acetylaminofluorene to rat liver DNA in vivo and consideration of the mechanism of binding of N-hydroxy-2-acetylaminofluorene to rat liver nucleic acids. Cancer Res. 1969 Oct;29(10):1799–1804. [PubMed] [Google Scholar]
- Jung G., Breitmaier E., Voelter W. Dissoziationsgleichgewichte von Glutathion. Eine Fourier-Transform- 13 C-NMR spektroskopische Untersuchung der PH-Abhängigkeit der Ladungsverteilung. Eur J Biochem. 1972 Jan 21;24(3):438–445. doi: 10.1111/j.1432-1033.1972.tb19704.x. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F., Ketterer B., Flammang T. J., Christodoulides L. Formation of 3-(glutathion-S-YL)-N-methyl-4-aminoazobenzene and inhibition of aminoazo dye-nucleic acid binding in vitro by reaction of glutathione with metabolically-generated N-methyl-4-aminoazobenzene-N-sulfate. Chem Biol Interact. 1980 Sep;31(3):265–278. doi: 10.1016/0009-2797(80)90015-0. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F., Miller J. A., Miller E. C. Hepatic metabolism of N-hydroxy-N-methyl-4-aminoazobenzene and other N-hydroxy arylamines to reactive sulfuric acid esters. Cancer Res. 1976 Jul;36(7 Pt 1):2350–2359. [PubMed] [Google Scholar]
- Keen J. H., Habig W. H., Jakoby W. B. Mechanism for the several activities of the glutathione S-transferases. J Biol Chem. 1976 Oct 25;251(20):6183–6188. [PubMed] [Google Scholar]
- Ketterer B., Beale D., Meyer D. The structure and multiple functions of glutathione transferases. Biochem Soc Trans. 1982 Apr;10(2):82–84. doi: 10.1042/bst0100082. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Christodoulides L. Two specific azodye-carcinogen-binding proteins of the rat liver. The identity of amino acid residues which bind the azodye. Chem Biol Interact. 1969 Dec;1(2):173–183. doi: 10.1016/0009-2797(69)90005-2. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Kadlubar F., Flammang T., Carne T., Enderby G. Glutathione adducts of N-methyl-4-aminoazobenzene formed in vivo and by reaction of N-benzoyloxy-N-methyl-4-aminoazobenzene with glutathione. Chem Biol Interact. 1979 Apr;25(1):7–21. doi: 10.1016/0009-2797(79)90065-6. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Ross-Mansell P., Whitehead J. K. The isolation of carcinogen-binding protein from livers of rats given 4-dimethylaminoazobenzene. Biochem J. 1967 May;103(2):316–324. doi: 10.1042/bj1030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer B., Srai S. K., Waynforth B., Tullis D. L., Evans F. E., Kadlubar F. F. Formation of N-(glutathion-S-methylene)-4-aminoazobenzene following metabolic oxidation of the N-methyl group of the carcinogen, N-methyl-4-aminoazobenzene. Chem Biol Interact. 1982 Feb;38(3):287–302. doi: 10.1016/0009-2797(82)90059-x. [DOI] [PubMed] [Google Scholar]
- Ketterer B. The role of nonenzymatic reactions of glutathione in xenobiotic metabolism. Drug Metab Rev. 1982;13(1):161–187. doi: 10.3109/03602538209002234. [DOI] [PubMed] [Google Scholar]
- King C. M., Phillips B. Enzyme-catalyzed reactions of the carcinogen N-hydroxy-2-fluorenylacetamide with nucleic acid. Science. 1968 Mar 22;159(3821):1351–1353. doi: 10.1126/science.159.3821.1351. [DOI] [PubMed] [Google Scholar]
- King H. W., Thompson M. H., Brookes P. The role of 9-hydroxybenzo(a)pyrene in the microsome mediated binding of benzo(a)pyrene to DNA. Int J Cancer. 1976 Sep 15;18(3):339–344. doi: 10.1002/ijc.2910180311. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- Kriek E. On the mechanism of action of carcinogenic aromatic amines. I. Binding of 2-acetylaminofluorene and N-hydroxy-2-acetylaminofluorene to rat-liver nucleic acids in vivo. Chem Biol Interact. 1969 Oct;1(1):3–17. doi: 10.1016/0009-2797(69)90015-5. [DOI] [PubMed] [Google Scholar]
- Litwack G., Ketterer B., Arias I. M. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature. 1971 Dec 24;234(5330):466–467. doi: 10.1038/234466a0. [DOI] [PubMed] [Google Scholar]
- McCray P. B., Gibson D. D., Fong K. L., Hornbrook K. R. Effect of glutathione peroxidase activity on lipid peroxidation in biological membranes. Biochim Biophys Acta. 1976 Jun 22;431(3):459–468. [PubMed] [Google Scholar]
- Meerman J. H., Beland F. A., Ketterer B., Srai S. K., Bruins A. P., Mulder G. J. Identification of glutathione conjugates formed from N-hydroxy-2-acetylaminofluorene in the rat. Chem Biol Interact. 1982 Mar 15;39(2):149–168. doi: 10.1016/0009-2797(82)90118-1. [DOI] [PubMed] [Google Scholar]
- Mitton J. R., Scholan N. A., Boyd G. S. The oxidation of cholesterol in rat liver sub-cellular particles. The cholesterol-7-alpha-Hydroxylase enzyme system. Eur J Biochem. 1971 Jun 29;20(4):569–579. doi: 10.1111/j.1432-1033.1971.tb01429.x. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., DePierre J. W., Ernster L. Activation of microsomal glutathione S-transferase activity by sulfhydryl reagents. Biochem Biophys Res Commun. 1979 Apr 13;87(3):657–663. doi: 10.1016/0006-291x(79)92009-6. [DOI] [PubMed] [Google Scholar]
- Mulder G. J., Unruh L. E., Evans F. E., Ketterer B., Kadlubar F. F. Formation and identification of glutathione conjugates from 2-nitrosofluorene and N-hydroxy-2-aminofluorene. Chem Biol Interact. 1982 Mar 1;39(1):111–127. doi: 10.1016/0009-2797(82)90010-2. [DOI] [PubMed] [Google Scholar]
- Müller A., Graf P., Wendel A., Sies H. Ethane production by isolated perfused rat liver: a system to study metabolic effects related to lipid peroxidation. FEBS Lett. 1981 Apr 20;126(2):241–244. doi: 10.1016/0014-5793(81)80251-7. [DOI] [PubMed] [Google Scholar]
- Nemoto N., Gelboin H. V., Habig W. H., Ketley J. N., Jakoby W. B. K region benzo (alpha) pyrene-4,5-oxide is conjugated by homogeneous gluthathione S-transferases. Nature. 1975 Jun 5;255(5508):512–512. doi: 10.1038/255512a0. [DOI] [PubMed] [Google Scholar]
- Nemoto N., Gelboin H. Assay and properties of glutathione-S-benzo(a)pyrene-4,5-oxide transferase. Arch Biochem Biophys. 1975 Oct;170(2):739–742. doi: 10.1016/0003-9861(75)90172-1. [DOI] [PubMed] [Google Scholar]
- Prohaska J. R., Ganther H. E. Glutathione peroxidase activity of glutathione-s-transferases purified from rat liver. Biochem Biophys Res Commun. 1976 May 23;76(2):437–445. doi: 10.1016/0006-291x(77)90744-6. [DOI] [PubMed] [Google Scholar]
- Rollins D. E., Buckpitt A. R. Liver cytosol catalyzed conjugation of reduced glutathione with a reactive metabolite of acetaminophen. Toxicol Appl Pharmacol. 1979 Feb;47(2):331–339. doi: 10.1016/0041-008x(79)90328-4. [DOI] [PubMed] [Google Scholar]
- Tipping E., Ketterer B., Christodoulides L., Enderby G. The non-convalent binding of small molecules by ligandin. Interactions with steroids and their conjugates, fatty acids, bromosulphophthalein carcinogens, glutathione and realted compounds. Eur J Biochem. 1976 Aug 16;67(2):583–590. doi: 10.1111/j.1432-1033.1976.tb10724.x. [DOI] [PubMed] [Google Scholar]
- Tipping E., Ketterer B. The influence of soluble binding proteins on lipophile transport and metabolism in hepatocytes. Biochem J. 1981 May 1;195(2):441–452. doi: 10.1042/bj1950441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel A., Feuerstein S., Konz K. H. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979 Jul 1;28(13):2051–2055. doi: 10.1016/0006-2952(79)90223-5. [DOI] [PubMed] [Google Scholar]


