Abstract
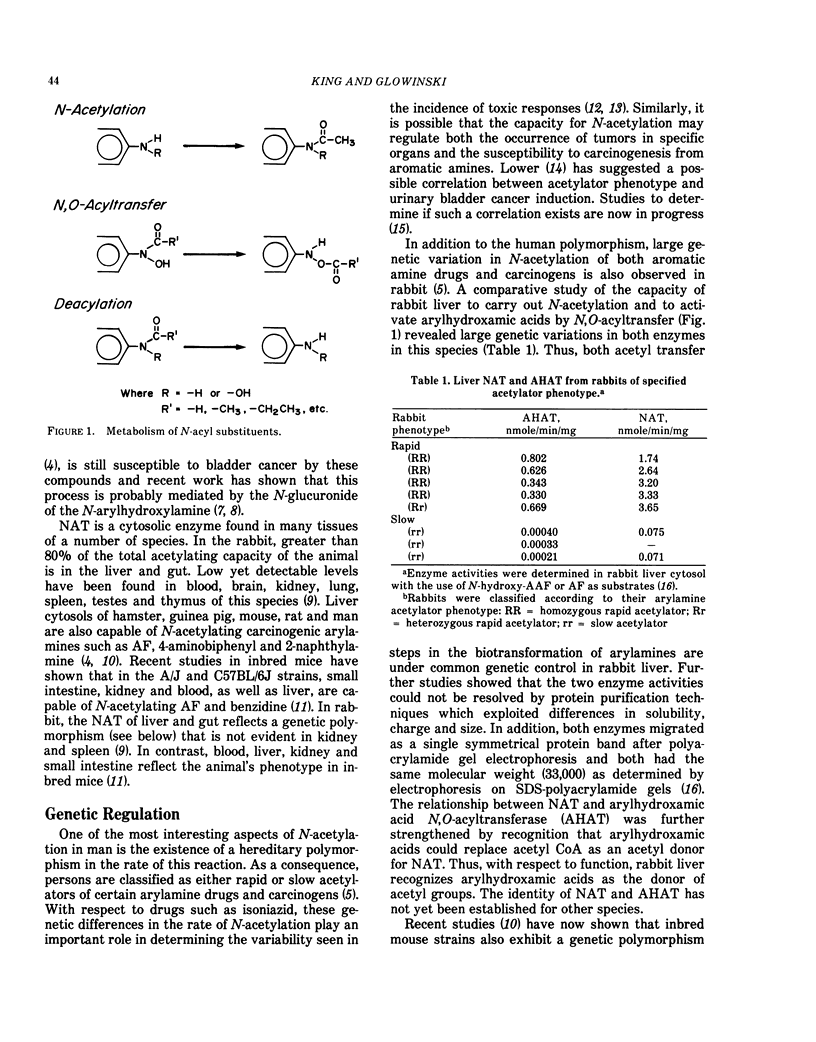
N-Substituted aromatic compounds can be metabolized in most species to N-acetylated derivatives that are themselves subject to further enzymatic transformations, including hydrolysis and N,O-acyltransfer. These proceses can either potentiate or ameliorate the biological responses to these N-substituted derivatives. Decreasing the levels of metabolites, such as arylhydroxylamines may, in some systems, reduce the probability of eliciting adverse biological effects. In others, arylhydroxamic acids produced by the acetylation of arylhydroxylamines may increase their potential for metabolic activation by N,O-acyltransfer. In the rabbit, rat and perhaps other species, the acetyl CoA-dependent N-acetyltransferase is also capable of activating arylhydroxamic acids by N-O-acyltransfer. These cytosolic organotriphosphate ester-resistant enzymes can utilize arylhydroxamic acid as a donor of the acetyl moiety in the acetyl transferase reaction and apparently are capable of activating arylhydroxamic acids because of their ability to O-acetylate the arylhydroxlamine. In mice, N-acetyltransferase and N,O-acetyltransferase seem not to exhibit this relationship. Enzymes from the microsomes of a number of species are also capable of activating arylhydroxamic acids. The particulate-bound enzymes are organotriphosphate ester-sensitive deacylases that are unable to form nucleic acid adducts on incubation with N-methoxy-N-acetylaminoarenes, substrates that are not capable of activation by N,O-acyltransfer. Thus, depending on the specificity of the enzymes involved, N-substituted aromatic compounds may be activated by N,O-acyltransfer during both the acetylation and deacylation process. The influence of this activation in the carcinogenic process is the object of continuing investigation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaben W. T., Weeks C. E., Weis C. C., Burger G. T., King C. M. Rat mammary gland carcinogenesis after local injection of N-hydroxy-N-acyl-2-aminofluorenes: relationship to metabolic activation. Carcinogenesis. 1982;3(3):233–240. doi: 10.1093/carcin/3.3.233. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Dworkin M., Miller J. A., Miller E. C. Electrophilic N-acetoxyaminoarenes derived from carcinogenic N-hydroxy-N-acetylaminoarenes by enzymatic deacetylation and transacetylation in liver. Biochim Biophys Acta. 1972 Dec 29;286(2):272–298. doi: 10.1016/0304-4165(72)90265-6. [DOI] [PubMed] [Google Scholar]
- Beland F. A., Allaben W. T., Evans F. E. Acyltransferase-mediated binding of N-hydroxyarylamides to nucleic acids. Cancer Res. 1980 Mar;40(3):834–840. [PubMed] [Google Scholar]
- Booth J. Acetyl transfer in arylamine metabolism. Biochem J. 1966 Sep;100(3):745–753. doi: 10.1042/bj1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona R. A., King C. M. Activation of the O-glucuronide of the carcinogen N-hydroxy-N-2-fluorenylacetamide by enzymatic deacetylation in vitro: formation of fluorenylamine-tRNA adducts. Biochem Pharmacol. 1976 May 1;25(9):1051–1056. doi: 10.1016/0006-2952(76)90495-0. [DOI] [PubMed] [Google Scholar]
- DeBaun J. R., Rowley J. Y., Miller E. C., Miller J. A. Sulfotransferase activation of N-hydroxy-2-acetylaminofluorene in rodent livers susceptible and resistant to this carcinogen. Proc Soc Exp Biol Med. 1968 Oct;129(1):268–273. doi: 10.3181/00379727-129-33301. [DOI] [PubMed] [Google Scholar]
- Drayer D. E., Reidenberg M. M. Clinical consequences of polymorphic acetylation of basic drugs. Clin Pharmacol Ther. 1977 Sep;22(3):251–258. doi: 10.1002/cpt1977223251. [DOI] [PubMed] [Google Scholar]
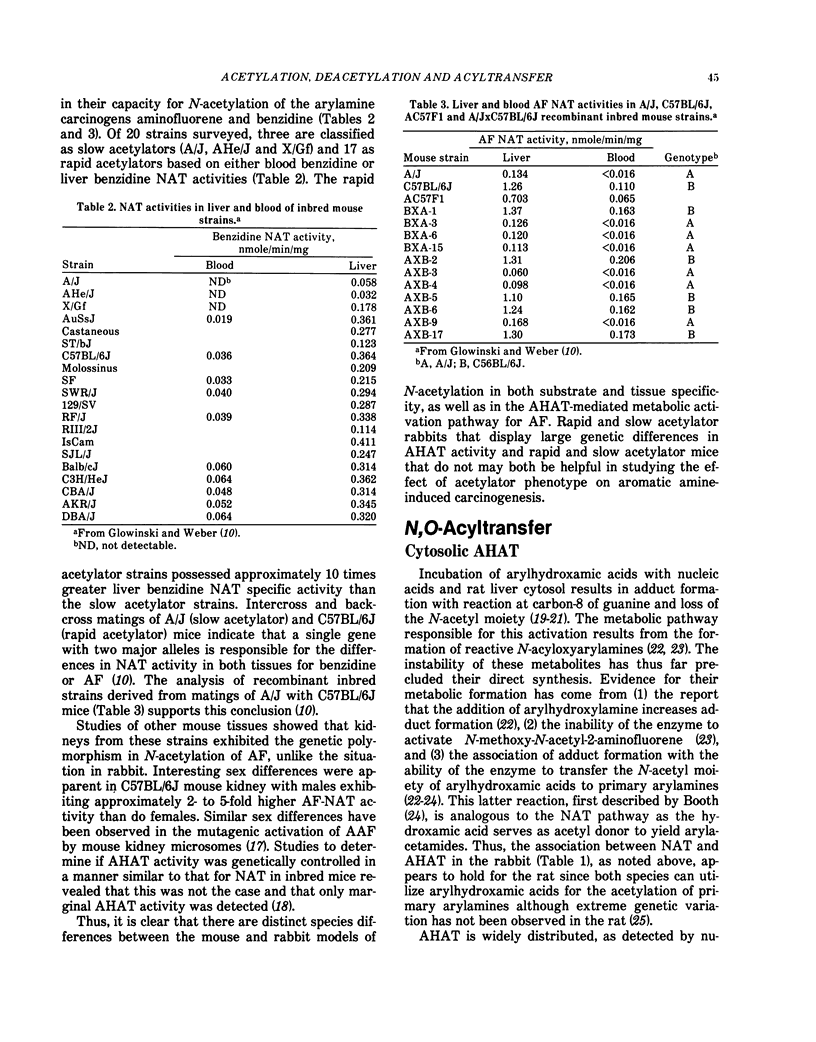
- Glowinski I. B., Radtke H. E., Weber W. W. Genetic variation in N-acetylation of carcinogenic arylamines by human and rabbit liver. Mol Pharmacol. 1978 Sep;14(5):940–949. [PubMed] [Google Scholar]
- Glowinski I. B., Weber W. W. Biochemical characterization of genetically variant aromatic amine N-acetyltransferases in A/J and C57BL/6J mice. J Biol Chem. 1982 Feb 10;257(3):1431–1437. [PubMed] [Google Scholar]
- Glowinski I. B., Weber W. W., Fysh J. M., Vaught J. B., King C. M. Evidence that arylhydroxamic acid N,O-acyltransferase and the genetically polymorphic N-acetyltransferase are properties of the same enzyme in rabbit liver. J Biol Chem. 1980 Aug 25;255(16):7883–7890. [PubMed] [Google Scholar]
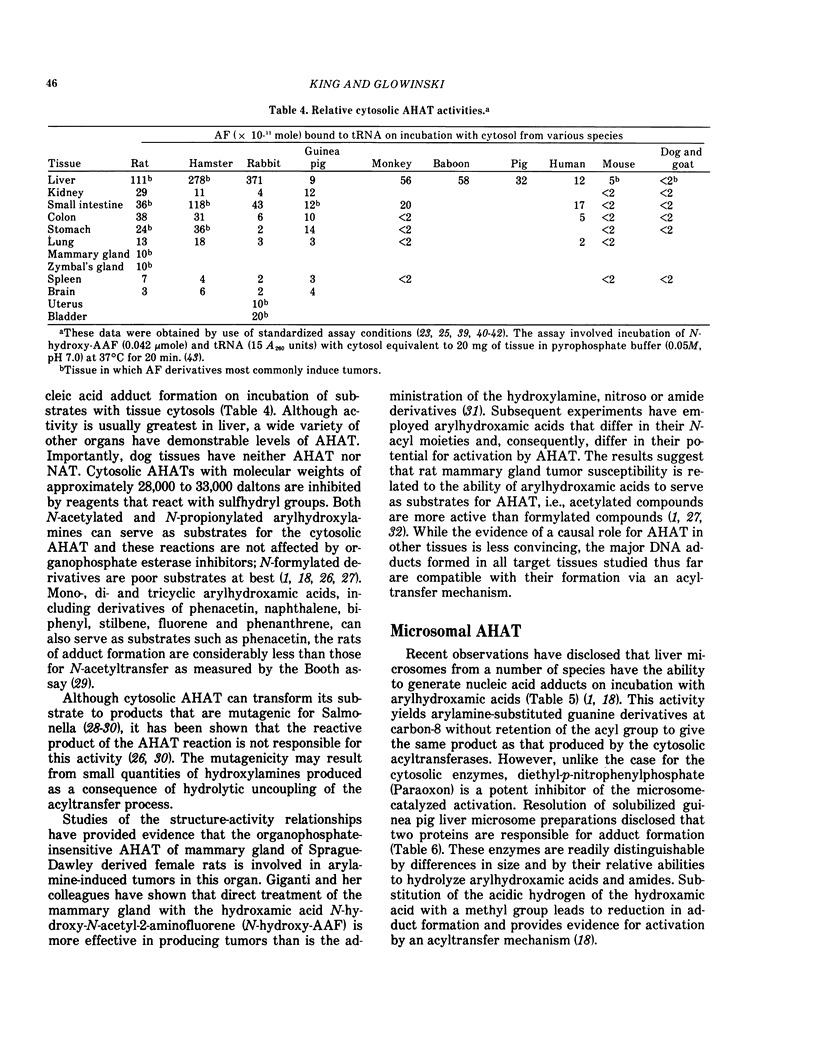
- Glowinski I. B., Weber W. W. Genetic regulation of aromatic amine N-acetylation in inbred mice. J Biol Chem. 1982 Feb 10;257(3):1424–1430. [PubMed] [Google Scholar]
- Hearse D. J., Weber W. W. Multiple N-acetyltransferases and drug metabolism. Tissue distribution, characterization and significance of mammalian N-acetyltransferase. Biochem J. 1973 Mar;132(3):519–526. doi: 10.1042/bj1320519. [DOI] [PMC free article] [PubMed] [Google Scholar]
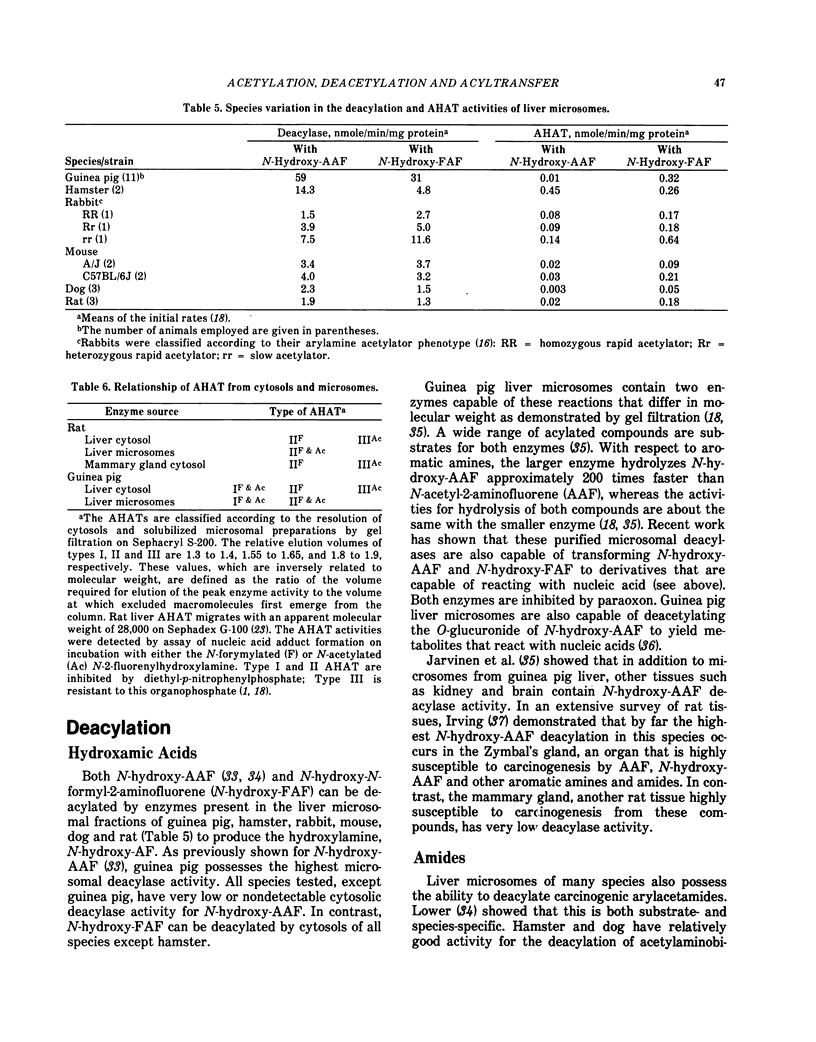
- Irving C. C. Enzymatic deacetylation of N-hydroxy-2-acetylaminofluorene by liver microsomes. Cancer Res. 1966 Jul;26(7):1390–1396. [PubMed] [Google Scholar]
- Järvinen M., Santti R. S., Hopsu-Havu V. K. Partial purification and characterization of two enzymes from guinea-pig liver microsomes that hydrolyze carcinogenic amides 2-acetylaminofluorene and N-hydroxy-2-acetylaminofluorene. Biochem Pharmacol. 1971 Nov;20(11):2971–2982. doi: 10.1016/0006-2952(71)90101-8. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F., Miller J. A., Miller E. C. Hepatic microsomal N-glucuronidation and nucleic acid binding of N-hydroxy arylamines in relation to urinary bladder carcinogenesis. Cancer Res. 1977 Mar;37(3):805–814. [PubMed] [Google Scholar]
- King C. M. Mechanism of reaction, tissue distribution, and inhibition of arylhydroxamic acid acyltransferase. Cancer Res. 1974 Jun;34(6):1503–1515. [PubMed] [Google Scholar]
- King C. M., Olive C. W., Cardona R. A. Activation of carcinogenic arylhydroxamic acids by human tissues. J Natl Cancer Inst. 1975 Aug;55(2):285–287. [PubMed] [Google Scholar]
- King C. M., Olive C. W. Comparative effects of strain, species, and sex on the acyltransferase- and sulfotransferase-catalyzed activations of N-hydroxy-N-2-fluorenylacetamide. Cancer Res. 1975 Apr;35(4):906–912. [PubMed] [Google Scholar]
- King C. M., Phillips B. Enzyme-catalyzed reactions of the carcinogen N-hydroxy-2-fluorenylacetamide with nucleic acid. Science. 1968 Mar 22;159(3821):1351–1353. doi: 10.1126/science.159.3821.1351. [DOI] [PubMed] [Google Scholar]
- King C. M., Traub N. R., Lortz Z. M., Thissen M. R. Metabolic activation of arylhydroxamic acids by N-O-acyltransferase of rat mammary gland. Cancer Res. 1979 Sep;39(9):3369–3372. [PubMed] [Google Scholar]
- Lotlikar P. D., Luha L. Enzymic N-acetylation of N-hydroxy-2-aminofluorene by liver cytosol from various species. Biochem J. 1971 Jun;123(2):287–289. doi: 10.1042/bj1230287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lower G. M., Jr, Bryan G. T. Enzymatic N-acetylation of carcinogenic aromatic amines by liver cytosol of species displaying different organ susceptibilities. Biochem Pharmacol. 1973 Jul 1;22(13):1581–1588. doi: 10.1016/0006-2952(73)90024-5. [DOI] [PubMed] [Google Scholar]
- Lower G. M., Jr, Bryan G. T. Enzymic deacetylation of carcinogenic arylacetamides by tissue microsomes of the dog and other species. J Toxicol Environ Health. 1976 Jan;1(3):421–432. doi: 10.1080/15287397609529341. [DOI] [PubMed] [Google Scholar]
- Lower G. M., Jr, Nilsson T., Nelson C. E., Wolf H., Gamsky T. E., Bryan G. T. N-acetyltransferase phenotype and risk in urinary bladder cancer: approaches in molecular epidemiology. Preliminary results in Sweden and Denmark. Environ Health Perspect. 1979 Apr;29:71–79. doi: 10.1289/ehp.792971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde P. K., Frislid K., Hansteen V. Disease and acetylation polymorphism. Clin Pharmacokinet. 1977 May-Jun;2(3):182–197. doi: 10.2165/00003088-197702030-00003. [DOI] [PubMed] [Google Scholar]
- Malejka-Giganti D., Gutmann H. R. N-hydroxy-2-fluorenylacetamide, an active intermediate of the mammary carcinogen N-hydroxy-2-fluorenylbenzenesulfonamide. Proc Soc Exp Biol Med. 1975 Oct;150(1):92–97. doi: 10.3181/00379727-150-38980. [DOI] [PubMed] [Google Scholar]
- Morton K. C., King C. M., Baetcke K. P. Metabolism of benzidine to N-hydroxy-N,N'-diacetylbenzidine and subsequent nucleic acid binding and mutagenicity. Cancer Res. 1979 Aug;39(8):3107–3113. [PubMed] [Google Scholar]
- NAGASAWA H. T., GUTMANN H. R. A note on the deacylation of the carcinogen 2-acetamidofluorene and related compounds by rat liver and intestine. Biochim Biophys Acta. 1957 Jul;25(1):186–189. doi: 10.1016/0006-3002(57)90439-0. [DOI] [PubMed] [Google Scholar]
- PETERS J. H., GUTMANN H. R. The acetylation of 2-aminofluorene and the deacetylation and concurrent reacetylation of 2-acetylaminofluorene by rat liver slices. J Biol Chem. 1955 Oct;216(2):713–726. [PubMed] [Google Scholar]
- POIRIER L. A., MILLER J. A., MILLER E. C. The N- and ring-hydroxylation of 2-acetylaminofluorene and the failure to detect N-acetylation of 2-aminofluorene in the dog. Cancer Res. 1963 Jun;23:790–800. [PubMed] [Google Scholar]
- Radomski J. L., Hearn W. L., Radomski T., Moreno H., Scott W. E. Isolation of the glucuronic acid conjugate of n-hydroxy-4-aminobiphenyl from dog urine and its mutagenic activity. Cancer Res. 1977 Jun;37(6):1757–1762. [PubMed] [Google Scholar]
- Shirai T., Fysh J. M., Lee M. S., Vaught J. B., King C. M. Relationship of metabolic activation of N-hydroxy-N-acylarylamines to biological response in the liver and mammary gland of the female CD rat. Cancer Res. 1981 Nov;41(11 Pt 1):4346–4353. [PubMed] [Google Scholar]
- Vaught J. B., McGarvey P. B., Lee M. S., Garner C. D., Wang C. Y., Linsmaier-Bednar E. M., King C. M. Activation of N-hydroxyphenacetin to mutagenic and nucleic acid-binding metabolites by acyltransfer, deacylation, and sulfate conjugation. Cancer Res. 1981 Sep;41(9 Pt 1):3424–3429. [PubMed] [Google Scholar]
- Weber W. W., King C. M. N-Acetyltransferase and arylhydroxamic acid acyltransferase. Methods Enzymol. 1981;77:272–280. doi: 10.1016/s0076-6879(81)77037-x. [DOI] [PubMed] [Google Scholar]
- Weeks C. E., Allaben W. T., Louie S. C., Lazear E. J., King C. M. Role of arylhydroxamic acid acyltransferase in the mutagenicity of N-hydroxy-N-2-fluorenylacetamide in Salmonella typhimurium. Cancer Res. 1978 Mar;38(3):613–618. [PubMed] [Google Scholar]
- Weeks C. E., Allaben W. T., Tresp N. M., Louie S. C., Lazear E. J., King C. M. Effects of structure of N-acyl-N-2-fluorenylhydroxylamines on arylhydroxamic acid acyltransferase, sulfotransferase, and deacylase activities, and on mutations in Salmonella typhimurium TA 1538. Cancer Res. 1980 Apr;40(4):1204–1211. [PubMed] [Google Scholar]


