Abstract
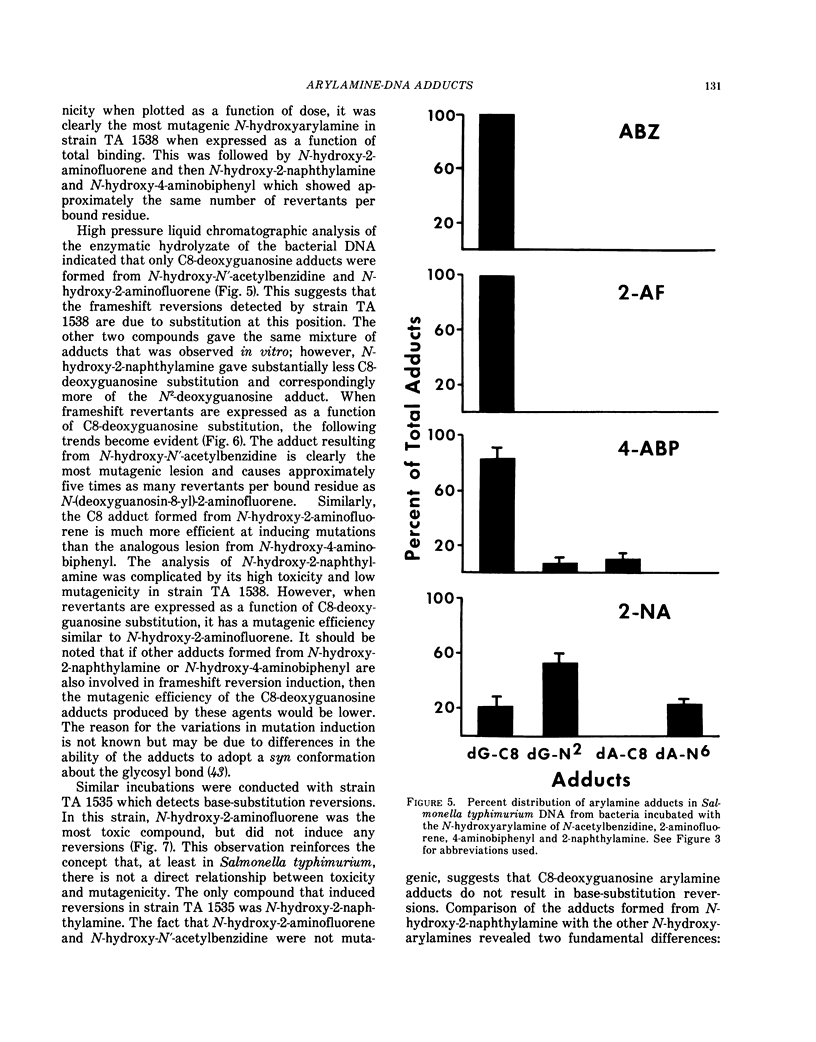
Hepatic N-oxidation, followed by N-glucuronidation, has been proposed as a route of metabolic activation for arylamine bladder carcinogens. It is postulated that the N-glucuronides are transported to the bladder lumen where they are hydrolyzed under slightly acidic conditions to release direct-acting carcinogenic and mutagenic N-hydroxyarylamines. In this study, 4-aminobiphenyl (ABP), 1-naphthylamine (1-NA), 2-naphthylamine (2-NA), 2-acetylaminofluorene (AAF), 4-nitrobiphenyl (NBP), benzidine (BZ), and N-acetylbenzidine (ABZ) were administered to male beagle dogs (60 μmole/kg), and the bladder epithelium DNA adducts were quantified at various times after treatment. At 24-48 hr after administration, the order of binding to bladder epithelium DNA was: ABP >> AAF > NBP ≅ 2-NA≅BZ ≅ ABZ >> 1-NA. The level of DNA modification by ABP remained constant for 7 days, whereas 2-NA and AAF residues decreased by 35% and 80%, respectively. The extent and relative persistence of total DNA binding correlated with the compounds' ability to induce bladder tumors in dogs. ABP, AAF, NBP, 2-NA and ABZ administration resulted in DNA binding sufficient for adduct analysis. Enzymatic hydrolysis of the DNA and examination of the adducts by high pressure liquid chromatography indicated that arylamine substitution at C8 of deoxyguanosine was the dominant product. Additional adducts were detected in animals treated with ABP, NBP, and 2-NA. Furthermore, the profiles of adducts obtained in vivo were remarkably similar to the profiles obtained when the N-hydroxy arylamine metabolites of these carcinogens were reacted with DNA in vitro at pH 5.0. To evaluate the mutagenic potential of these arylamine-DNA adducts, Salmonella typhimurium strains TA 1535 and TA 1538 were incubated with N-hydroxy-2-NA, N-hydroxy-2-aminofluorene (AF), N-hydroxy-ABP, and N-hydroxy-ABZ and the resulting DNA adducts and reversions were quantified. Arylamine-C8-deoxyguanosine substitution was correlated with frameshift reversions induced by these agents, with the lesions showing a relative order of mutagenic efficiency of ABZ>AF≅2-NA>ABP. These data suggest that mutagenic N-hydroxyarylamines may be ultimate carcinogens for the bladder epithelium. Furthermore, if one assumes that a mutagenic lesion is important for tumor initiation, then C8-deoxyguanosine substitution by these compounds may be significant for urinary bladder carcinogenesis.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beland F. A., Allaben W. T., Evans F. E. Acyltransferase-mediated binding of N-hydroxyarylamides to nucleic acids. Cancer Res. 1980 Mar;40(3):834–840. [PubMed] [Google Scholar]
- Beland F. A., Dooley K. L., Casciano D. A. Rapid isolation of carcinogen-bound DNA and RNA by hydroxyapatite chromatography. J Chromatogr. 1979 Jun 1;174(1):177–186. doi: 10.1016/s0021-9673(00)87048-x. [DOI] [PubMed] [Google Scholar]
- Beranek D. T., White G. L., Heflich R. H., Beland F. A. Aminofluorene-DNA adduct formation in Salmonella typhimurium exposed to the carcinogen N-hydroxy-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5175–5178. doi: 10.1073/pnas.79.17.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. J., Brookes P. Lack of oestradiol-DNA binding in mouse embryo cell cultures or following rat-liver microsomal metabolism. Cancer Lett. 1979 May;6(6):351–355. doi: 10.1016/s0304-3835(79)80093-2. [DOI] [PubMed] [Google Scholar]
- Glowinski I. B., Radtke H. E., Weber W. W. Genetic variation in N-acetylation of carcinogenic arylamines by human and rabbit liver. Mol Pharmacol. 1978 Sep;14(5):940–949. [PubMed] [Google Scholar]
- Kadlubar F. F., Anson J. F., Dooley K. L., Beland F. A. Formation of urothelial and hepatic DNA adducts from carcinogen 2-naphthylamine. Carcinogenesis. 1981;2(5):467–470. doi: 10.1093/carcin/2.5.467. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F., Miller J. A., Miller E. C. Guanyl O6-arylamination and O6-arylation of DNA by the carcinogen N-hydroxy-1-naphthylamine. Cancer Res. 1978 Nov;38(11 Pt 1):3628–3638. [PubMed] [Google Scholar]
- Kadlubar F. F., Miller J. A., Miller E. C. Hepatic microsomal N-glucuronidation and nucleic acid binding of N-hydroxy arylamines in relation to urinary bladder carcinogenesis. Cancer Res. 1977 Mar;37(3):805–814. [PubMed] [Google Scholar]
- Kadlubar F. F., Unruh L. E., Flammang T. J., Sparks D., Mitchum R. K., Mulder G. J. Alteration of urinary levels of the carcinogen, N-hydroxy-2-naphthylamine, and its N-glucuronide in the rat by control of urinary pH, inhibition of metabolic sulfation, and changes in biliary excretion. Chem Biol Interact. 1981 Jan;33(2-3):129–147. doi: 10.1016/0009-2797(81)90036-3. [DOI] [PubMed] [Google Scholar]
- King C. M., Olive C. W. Comparative effects of strain, species, and sex on the acyltransferase- and sulfotransferase-catalyzed activations of N-hydroxy-N-2-fluorenylacetamide. Cancer Res. 1975 Apr;35(4):906–912. [PubMed] [Google Scholar]
- King C. M., Phillips B. N-hydroxy-2-fluorenylacetamide. Reaction of the carcinogen with guanosine, ribonucleic acid, deoxyribonucleic acid, and protein following enzymatic deacetylation or esterification. J Biol Chem. 1969 Nov 25;244(22):6209–6216. [PubMed] [Google Scholar]
- Kriek E. On the interaction of N-2-fluorenylhydroxylamine with nucleic acids in vitro. Biochem Biophys Res Commun. 1965 Sep 22;20(6):793–799. doi: 10.1016/0006-291x(65)90088-4. [DOI] [PubMed] [Google Scholar]
- Kriek E., Westra J. G. Structural identification of the pyrimidine derivatives formed from N-(deoxyguanosin-8-yl)-2-aminofluorene in aqueous solution at alkaline pH. Carcinogenesis. 1980 Jun;1(6):459–468. doi: 10.1093/carcin/1.6.459. [DOI] [PubMed] [Google Scholar]
- Lower G. M., Jr, Bryan G. T. Enzymatic N-acetylation of carcinogenic aromatic amines by liver cytosol of species displaying different organ susceptibilities. Biochem Pharmacol. 1973 Jul 1;22(13):1581–1588. doi: 10.1016/0006-2952(73)90024-5. [DOI] [PubMed] [Google Scholar]
- Lower G. M., Jr, Bryan G. T. Enzymic deacetylation of carcinogenic arylacetamides by tissue microsomes of the dog and other species. J Toxicol Environ Health. 1976 Jan;1(3):421–432. doi: 10.1080/15287397609529341. [DOI] [PubMed] [Google Scholar]
- Lower G. M., Jr, Nilsson T., Nelson C. E., Wolf H., Gamsky T. E., Bryan G. T. N-acetyltransferase phenotype and risk in urinary bladder cancer: approaches in molecular epidemiology. Preliminary results in Sweden and Denmark. Environ Health Perspect. 1979 Apr;29:71–79. doi: 10.1289/ehp.792971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry L. K., Tolos W. P., Boeniger M. F., Nony C. R., Bowman M. C. Chemical monitoring of urine from workers potentially exposed to benzidine-derived azo dyes. Toxicol Lett. 1980 Nov;7(1):29–36. doi: 10.1016/0378-4274(80)90081-8. [DOI] [PubMed] [Google Scholar]
- MORRIS H. P., EYESTONE W. H. Tumors of the liver and urinary bladder of the dog after ingestion of 2-acetylaminofluorene. J Natl Cancer Inst. 1953 Apr;13(5):1139–1165. [PubMed] [Google Scholar]
- Martin C. N., Beland F. A., Roth R. W., Kadlubar F. F. Covalent binding of benzidine and N-acetylbenzidine to DNA at the C-8 atom of deoxyguanosine in vivo and in vitro. Cancer Res. 1982 Jul;42(7):2678–2686. [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDONALD D. F., LUND R. R. The role of the urine in vesical neoplasm. I. Experimental confirmation of the urogenous theory of pathogenesis. J Urol. 1954 May;71(5):560–570. doi: 10.1016/S0022-5347(17)67826-1. [DOI] [PubMed] [Google Scholar]
- Metzger G., Werbin H. Evidence for N-acetoxy-N-2-acetylaminofluorene induced covalent-like binding of some nonhistone proteins to DNA in chromatin. Biochemistry. 1979 Feb 20;18(4):655–659. doi: 10.1021/bi00571a016. [DOI] [PubMed] [Google Scholar]
- Mirsalis J. C., Hamm T. E., Jr, Sherrill J. M., Butterworth B. E. Role of gut flora in the genotoxicity of dinitrotoluene. Nature. 1982 Jan 28;295(5847):322–323. doi: 10.1038/295322a0. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Koreeda M. Application of the change in partition coefficient with pH to the structure determination of alkyl substituted guanosines. Biochem Biophys Res Commun. 1976 Nov 22;73(2):459–464. doi: 10.1016/0006-291x(76)90729-4. [DOI] [PubMed] [Google Scholar]
- Oldham J. W., Kadlubar F. F., Milo G. E. Effect of pH on the neoplastic transformation of normal human skin fibroblasts by N-hydroxy-1-naphthylamine and N-hydroxy-2-naphthylamine. Carcinogenesis. 1981;2(9):937–940. doi: 10.1093/carcin/2.9.937. [DOI] [PubMed] [Google Scholar]
- POIRIER L. A., MILLER J. A., MILLER E. C. The N- and ring-hydroxylation of 2-acetylaminofluorene and the failure to detect N-acetylation of 2-aminofluorene in the dog. Cancer Res. 1963 Jun;23:790–800. [PubMed] [Google Scholar]
- Poirier L. A., de Serres F. J. Initial National Cancer Institute studies on mutagenesis as a prescreen for chemical carcinogens: an appraisal. J Natl Cancer Inst. 1979 Apr;62(4):919–926. [PubMed] [Google Scholar]
- Poupko J. M., Hearn W. L., Radomski J. L. N-Glucuronidation of N-hydroxy aromatic amines: a mechanism for their transport and bladder-specific carcinogenicity. Toxicol Appl Pharmacol. 1979 Sep 30;50(3):479–484. doi: 10.1016/0041-008x(79)90401-0. [DOI] [PubMed] [Google Scholar]
- Purchase I. F., Kalinowski A. E., Ishmael J., Wilson J., Gore C. W., Chart I. S. Lifetime carcinogenicity study of 1- and 2-naphthylamine in dogs. Br J Cancer. 1981 Dec;44(6):892–901. doi: 10.1038/bjc.1981.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski J. L. The primary aromatic amines: their biological properties and structure-activity relationships. Annu Rev Pharmacol Toxicol. 1979;19:129–157. doi: 10.1146/annurev.pa.19.040179.001021. [DOI] [PubMed] [Google Scholar]
- SCOTT W. W., BOYD H. L. A study of the carcinogenic effect of beta-naphthylamine on the normal and substituted isolated sigmoid loop bladder of dogs. J Urol. 1953 Dec;70(6):914–925. doi: 10.1016/S0022-5347(17)68005-4. [DOI] [PubMed] [Google Scholar]


