Protein misfolding is now recognized to be a major contributing factor in a number of protein folding diseases, including amyotropic laterial sclerosis, cystic fibrosis, Alzheimer's disease, Parkinson's disease, and a host of many different amyloidosis diseases. Protein aggregation and misfolding reactions are also the bane of protein production and impede pharmaceutical drug development. Understanding the fundamental in vivo factors that control the kinetics of protein misfolding is the crucial aspect involved in developing procedures and strategies to avoid this deleterious side reaction. Elegant in vivo work of Nollen et al. (1) has shown that the elements controlling protein homeostasis such as protein synthesis, energy production in the cell, chaperone-dependent protein folding, protein transport, and protein degradation collectively control intracellular protein aggregation. However, it is also known that protein folding is influenced by the presence of intracellular osmolytes that, in turn, dramatically affect protein stabilities, protein folding rates, and protein aggregation. Thus, it would be highly useful if we could determine how osmolytes directly influence the kinetics of protein folding and aggregation in vivo. In this issue of PNAS, Ignatova and Gierasch (2) accomplish this feat by using their earlier in vivo protein aggregation cellular retinoic acid binding protein (CRABP) 4′,5′-bis(1,3,2-dithioarsolan-2-yl)fluorescein (FlAsH) model system (3) to monitor in vivo formation of amorphous and fibrillar/amyloid-like aggregation reactions driven by either misfolding or polyglutamine (53htt) aggregation in the presence of high intracellular proline concentrations. Interestingly, they find that the aggregation propensities and kinetics of their particular folding variants of CRABP FlAsH are dramatically altered when proline concentration levels are changed before and during the aggregation reaction (Fig. 1). This system allows these investigators to directly visualize for the first time the in vivo effects of rapid accumulation of an intracellular osmolyte during protein aggregation.
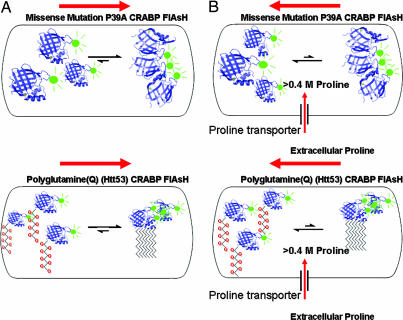
Fig. 1.
Ignatova and Gierasch (2) have constructed the P39A CRABP–FlAsH and polyQ Htt53 CRABP–FlAsH model systems to monitor the kinetic progress of in vivo protein aggregation, thus allowing them to directly follow the generation of amorphous or fibrillar protein aggregation caused either by protein misfolding or amyloid formation (A, Upper and Lower, respectively). The aggregation was easily observed through the development of distinct fluorescent foci within the cell. By rapidly increasing the intracellular concentration of the naturally occurring osmolyte proline (B) using an up-regulation of the ProP (proline transporter), these investigators were able to monitor, for the first time, both the inhibition of aggregation and the shift in the dynamics on the real-time kinetics of disaggregation of amorphous and fibrillar aggregations in response to a substantial increase in intracellular proline concentrations (B). These experiments demonstrate a potentially easy methodology to rapidly control intracellular aggregation reactions simply by rapidly increasing the concentration of a particular intracellular osmolyte.
Naturally occurring osmolytes have been selected by evolution to accumulate in response to intracellular protein aggregation caused by desiccation, heat stress, freezing, high hydrostatic pressure, and the transient or constitutive increase in intracellular denaturants such as urea (4). To explore how osmolytes exert different effects on protein folding and stability, Bolen and Baskakov (5) measured the transfer free energies of amino acid side chains and peptide backbone model compounds by measuring the solubility difference of these particular model compounds in either water or water/osmolyte solutions. This free energy approach provides a particularly satisfying energetic rationale of the differential stabilization effects that various osmolytes have on protein structure, stability folding, and aggregation (5, 6). Most importantly, their analysis indicates that the peptide backbone is preferentially buried in the presence of specific osmolytes, providing a reasonable thermodynamic argument to explain why one observes increases in protein stability and folding with stabilizing or protecting osmolytes. Protecting osmolytes are defined as those that increase protein folding and stability. When the transfer free energies of all of the amino acid side chains and entire peptide backbone of a protein are summed and compared, the protecting osmolytes influence protein stability over a range of free energies. For instance, adding the natural osmolyte trimethylamine N oxide (TMAO) to a protein solution often results in the largest free energy increase in protein stability and protein folding collapse. The increase in protein stability is directly correlated with the large unfavorable decrease in the peptide backbone stability, resulting in a large destabilization of protein states that expose large surface areas of peptide backbone. In contrast, the addition of proline to protein solutions seems to result in only a modest increase in protein stability and collapse. However, because proline preferentially interacts with the side chains of proteins in addition to the unfavorable backbone transfer free energy (6) the sum of these free energies provides plausible energetic reasons why proline disfavors protein aggregation during folding (7). Deciding which osmolyte one chooses to inhibit or prevent general protein aggregation is critical because it is well documented that some of the stronger osmolytes such as TMAO and even glycerol actually facilitate premature protein collapse, sometimes leading to accelerated aggregation and amyloid formation for a number of intrinsically unstructured proteins (8, 9). With their established aggregation system, Ignatova and Gierasch (2, 3) can determine what happens to in vivo protein aggregation reaction kinetics during a rapid increase of the intracellular proline pool. To accomplish this feat, they used an Escherichia coli strain with a highly controllable expression of the proline transporter (ProP) developed in the laboratory of Janet Wood (10). The induction of the proline transporter coupled with salt-induced activation and a rapid influx of proline from available extracellular pools results in an increase in the intracellular concentration of proline >0.4 M.
What makes this work particularly interesting is the observation that there is excellent agreement between the kinetics of aggregation both in vivo and in vitro. When proline addition or uptake is delayed during either in vitro or in vivo protein aggregation, very similar inhibition or aggregation kinetic profiles were observed. For the polyglutamine chimer tetra-Cys CRABP httQ53-forming fibrillar aggregates, early proline addition significantly inhibits the initial aggregation reactions both in vivo and in vitro.
One of the holy grails in understanding the molecular basis of protein aggregation diseases lies in identifying, characterizing, and controlling the formation of the partially folded soluble prefibrillar aggregates (11). These elusive soluble forms may be critical players in cellular toxicity, and recent efforts of many amyloid researchers have focused on finding conditions where the formation of these species can be stabilized or kinetically controlled. Therefore, it would be extremely interesting to determine whether the decreased aggregate formation is accompanied by the concomitant increase in these elusive cytotoxic species. In this way, the facile control or enhanced accumulation of these aggregating species could be extremely important in the development of small-molecule “pharmacological chaperones” (12). Consequently, it is exciting to speculate as the authors do that the osmolytes may change the overall character of the initial aggregating species. If this notion turns out to be true, one could use these defined conditions to develop small-molecule inhibitors against these detergent-liable populations. Even under circumstances where the proline-induced formation of an easily identifiable prefibrillar species may not be realized, it is nonetheless intriguing that the aggregation reaction can be easily controlled at the outset. Certainly more physicochemical characterizations of these transient smaller aggregates are warranted.
Osmolyte proline similarly affects the kinetics of in vitro and in vivo protein misfolding.
Inevitably, constructing a system that can easily control the in vivo concentrations of a stabilizing and solubilizing osmolyte has many other practical uses. By way of example, it is estimated that ≈50% of the human diseases are caused by folding defects. Although a large number of these folding defects can potentially be rescued by binding small-molecule therapeutic ligands to the native fold, one still does not have an easy method for identifying which of the many missense protein folding mutations would be good candidates for targeting therapies with pharmacological small-molecule chaperones. Indeed, because osmolytes such as trimethylamine N oxide and glycerol can rescue the folding defect of the Δ F508 mutant of the cystic fibrosis transmembrane regulator, the simple fact that this mutant can be folded to a stable native-like conformation (13) forms the critical basis behind developing small-molecule strategies for treating this particular protein folding disease. In the more general case, by expanding this ability to control osmolyte concentrations in vivo, one can solubilize and stabilize a host of overexpressed folding mutants for any particular protein folding disease state, thus easily identifying protein folding mutants with an intrinsic ability to fold. As an example, Song and Chuang (14) used an in vitro chaperonin/osmolyte combination to demonstrate that the folding/assembly mutation of α-ketoacid dehydrogenase that causes maple syrup urine disease can be reversed using a combination of folding aids. Once folded, the protein remained stable, suggesting that this particular mutation may be part of the misfolding class of proteins that resemble the temperature-sensitive folding mutants (15). This missense folding mutation may be an excellent candidate for small-molecule therapeutic rescue.
To expand on this system, it is entirely possible that in vivo effects of proline concentration control could be further enhanced by increasing other in vivo folding assistants in a synergistic manner. For example, osmolyte-enhanced folding/antiaggregation could be further augmented by the simultaneous increase in select molecular chaperones, particularly those involved in folding. From a biotechnology prospective, using in vitro and in vivo osmolyte/chaperone protein combinations could also result in a dramatic increase in the levels of correctly folded proteins (16–18). Alternatively, one could examine the possibility that combinations of osmolytes may also facilitate protein folding. Increases in other naturally occurring osmolytes such as glycine betaine have been shown to rescue protein misfolding in vivo (19). Many diverse intracellular osmolyte combinations could certainly be tried. For instance, proline along with other antiaggregation osmolytes such as trehalose (20) may be useful for determining how endogeneously synthesized intracellular osmolytes may act synergistically to more effectively prevent general in vivo protein aggregation. Enabling one to directly control the intracellular osmolyte conditions during in vivo protein folding is an extremely useful procedure that will enhance everyone's protein folding toolbox.
Footnotes
Conflict of interest statement: No conflicts declared.
See companion article on page 13357.
References
- 1.Nollen E. A. A., Garcia S. M., van Haaften G., Kim S., Chavez A., Morimoto R. I., Plasterk R. H. A. Proc. Natl. Acad. Sci. USA. 2004;101:6403–6408. doi: 10.1073/pnas.0307697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ignatova Z., Gierasch L. M. Proc. Natl. Acad. Sci. USA. 2006;103:13357–13361. doi: 10.1073/pnas.0603772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ignatova Z., Gierasch L. M. Proc. Natl. Acad. Sci. USA. 2004;101:523–528. doi: 10.1073/pnas.0304533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 5.Bolen D. W., Baskakov I. V. J. Mol. Biol. 2000;310:955–963. doi: 10.1006/jmbi.2001.4819. [DOI] [PubMed] [Google Scholar]
- 6.Auton M., Bolen D. W. Proc. Natl. Acad. Sci. USA. 2005;102:15065–15068. doi: 10.1073/pnas.0507053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuel D., Thallampuranam K., Kumar S., Ganesh G., Jayaraman G., Yang P.-W., Chang M.-M., Trivedi V. D., Wang S.-L, Hwang K.-C, et al. Protein Sci. 2000;9:344–352. doi: 10.1110/ps.9.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uversky V. N., Li J., Fink A. L. FEBS Lett. 2001;509:31–35. doi: 10.1016/s0014-5793(01)03121-0. [DOI] [PubMed] [Google Scholar]
- 9.Yang D. S., Yip C. M., Huang T. H., Chakrabartty A., Fraser P. E. J. Biol. Chem. 1999;274:32970–32974. doi: 10.1074/jbc.274.46.32970. [DOI] [PubMed] [Google Scholar]
- 10.Racher K. I., Culham D. E., Wood J. E. Biochemistry. 2001;40:7324–7433. doi: 10.1021/bi002331u. [DOI] [PubMed] [Google Scholar]
- 11.Stefani M., Dobson C. J. Mol. Med. 2003;81:678–699. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 12.Cohen F. E., Kelly J. W. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 13.Brown C. R., Hong-Brown L. Q., Welch W. J. J. Clin. Invest. 1997;99:1432–1444. doi: 10.1172/JCI119302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song J.-L., Chuang D. T. J. Biol. Chem. 2001;276:40241–40246. doi: 10.1074/jbc.M107242200. [DOI] [PubMed] [Google Scholar]
- 15.Mitraki A., King J. FEBS Lett. 1992;307:20–25. doi: 10.1016/0014-5793(92)80894-m. [DOI] [PubMed] [Google Scholar]
- 16.Diamant S., Eliahu N., Rosenthal D., Goloubinoff P. J. Biol. Chem. 2001;276:39586–39591. doi: 10.1074/jbc.M103081200. [DOI] [PubMed] [Google Scholar]
- 17.Voziyan P. A., Jadhav L., Fisher M. T. Pharm. Sci. 2001;89:1036–1045. doi: 10.1002/1520-6017(200008)89:8<1036::aid-jps8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Voziyan P. A., Johnston M., Chao A., Bomhoff G., Fisher M. T. J. Struct. Funct. Genomics. 2005;31:1–6. doi: 10.1007/s10969-005-2646-6. [DOI] [PubMed] [Google Scholar]
- 19.Bourot S., Sire O., Trautwetter A., Touze T., Wu L. F., Blanco C., Bernard T. J. Biol. Chem. 2000;275:1050–1056. doi: 10.1074/jbc.275.2.1050. [DOI] [PubMed] [Google Scholar]
- 20.Singer M. A., Lindquist S. Mol. Cell. 1998;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]