Abstract
Small organic molecules termed osmolytes are harnessed by a variety of cell types in a wide range of organisms to counter unfavorable physiological conditions that challenge protein stability and function. Using a well characterized reporter system that we developed to allow in vivo observations, we have explored how the osmolyte proline influences the stability and aggregation of a model aggregation-prone protein, P39A cellular retinoic acid-binding protein. Strikingly, we find that the natural osmolyte proline abrogates aggregation both in vitro and in vivo (in an Escherichia coli expression system). Importantly, proline also prevented aggregation of constructs containing exon 1 of huntingtin with extended polyglutamine tracts. Although compatible osmolytes are known to stabilize the native state, our results point to a destabilizing effect of proline on partially folded states and early aggregates and a solubilizing effect on the native state. Because proline is believed to act through a combination of solvophobic backbone interactions and favorable side-chain interactions that are not specific to a particular sequence or structure, the observed effect is likely to be general. Thus, the osmolyte proline may be protective against biomedically important protein aggregates that are hallmarks of several late-onset neurodegenerative diseases including Huntington’s, Alzheimer’s, and Parkinson’s. In addition, these results should be of practical importance because they may enable protein expression at higher efficiency under conditions where aggregation competes with proper folding.
Keywords: amyloid, osmolyte, polyglutamine, proline, Huntington’s disease
Harsh environmental conditions or normal physiological extremes lead to situations where cells are subjected to solutions of high osmolality, which causes loss of water, increased crowding, and consequent increased intracellular concentrations of both macromolecules and small solutes. Given that the concentration of macromolecules in the cell is already high (>250 mg/ml), maintenance of fidelity in critical reactions such as protein folding and retention of the functions of key macromolecules are challenged by osmotic stress. Many cells up-regulate the concentrations of specific small organic molecules termed osmolytes, compatible solutes, or osmoprotectants, either by de novo synthesis or import, to cope with loss of water (1–3). These species counteract the deleterious effects of high osmolality by their favorable interaction with solvent water and their unfavorable interactions with protein surfaces (a combination of backbone and side-chain interactions that varies from osmolyte to osmolyte) (4–7). Because a denatured state has a greater exposed backbone surface area than a folded native state, protecting osmolytes stabilize proteins. Their influence on other required processes, such as assembly reactions, enzyme activity, and complex formation, has been examined in detail in only a few cases, but the results are consistent with the general paradigm that their impact is solvophobic, and thus they will favor species with reduced solvent-accessible surface area. Their impact varies, however, because of their differing interactions with protein side chains. Proline has a pronounced ability to solubilize proteins (8) and a more modest stabilizing effect than other osmolytes (4, 7, 9), presumably because its favorable interactions with side chains exposed in the native state outweigh its solvophobic effect on the backbone. Significantly, proline has been reported to disfavor protein aggregation in vitro (10, 11).
Given this general model of action, it is of interest to ask how osmolytes influence protein aggregation. This process is of acute biomedical interest, given the recognition that several devastating pathologies, including the neurodegenerative diseases such as Huntington’s, Alzheimer’s, and Parkinson’s, are etiologically correlated with the deposition of aggregated protein (amyloid) either intracellularly or extracellularly (12–15). One could envision differing effects depending on both the mechanism of aggregation and the particular osmolyte: If aggregation is dominated by the destabilization of a native state by mutation, then protecting osmolytes would be expected to have ameliorative effects. The effect of an osmoprotectant could also be mediated by the nature of the obligatory nucleus that must form before aggregation proceeds via a nucleation-propagation process, and one could anticipate that the nucleus may be less compact than the native state, also leading to an expectation of reduced propensity for aggregation in the presence of the osmolyte. However, higher-order aggregates sequester considerable surface area and may be preferentially stabilized by osmolytes. To date, systematic studies of osmolyte effects on aggregation are lacking. However, several reports indeed suggest that osmolytes such as trehalose, ectoine, or tetramethylamine oxide may reduce the propensity of aggregation of biomedically important amyloidogenic proteins, including polyglutamine (polyQ)-containing species (correlated with Huntington’s and ≈9 other neurodegenerative diseases) (16–18), the β-amyloid peptide implicated in Alzheimer’s disease (19–22), and the well studied yeast prion (23). Conflicting results, however, emphasize the need for careful analysis of a well behaved system both in vitro and in vivo (19, 20, 24).
We have taken advantage of an experimental system that we designed to observe the in vivo stability and aggregation of a protein of interest (25). We use the protein cellular retinoic acid-binding protein (CRABP), modified to incorporate a Cys-Cys-Gly-Pro-Cys-Cys sequence that can bind the biarsenical fluoroscein derivative, 4′,5′-bis(1,3,2-dithioarsolan-2-yl)fluorescein (FlAsH) (26). We have demonstrated that the in vivo FlAsH signal reports on whether tetra-Cys CRABP is native or not. Moreover, when the FlAsH-binding motif was inserted into an aggregation-prone, slow-folding variant of CRABP with a Pro-to-Ala mutation at position 39, the FlAsH signal could be used to follow aggregation in vivo and in vitro (25, 27). Recently, we used this design as a basis to explore the impact of an adjacent polyQ tract [exon 1 of the protein huntingtin (Htt)] on the behavior of the stably folded CRABP in vivo (28). In the present study, we report that an osmotic stress response mediated by the osmolyte proline blocks aggregation of both P39A tetra-Cys CRABP and the chimera between tetra-Cys CRABP and Htt exon 1 (with a pathological glutamine repeat length of 53) both in vivo and in vitro when proline is present at early stages of aggregation and aggregate sizes are modest. Once larger aggregates have formed, the osmolyte no longer impedes aggregation. This finding is consistent with the expectation based on current physical chemical models for osmolyte action. Also, these results should help in the application of osmolytes to biomedical therapeutics or biotechnological processes.
Results
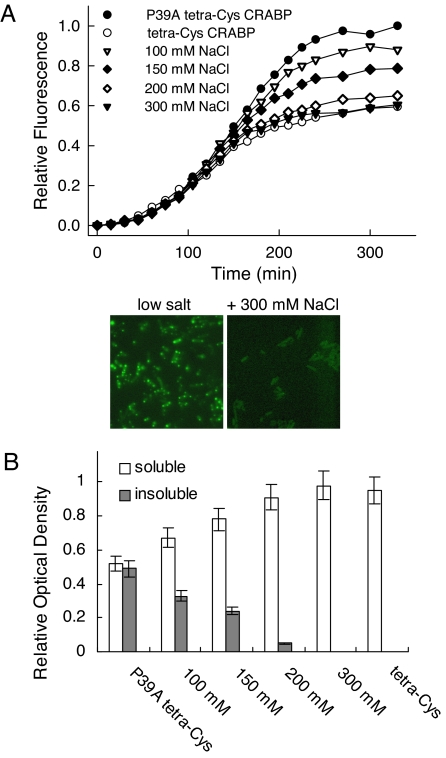
FlAsH fluorescence of cells expressing the aggregation-prone P39A tetra-Cys CRABP exposed to media of increasing osmolality showed profiles that approached that of the soluble tetra-Cys CRABP (Fig. 1A). We have previously shown that the fluorescence increase at later time points (as seen for low salt) indicates formation of a highly fluorescent aggregated state. Here, by 300 mM NaCl, cellular fractionation showed that the protein is fully soluble at all time points (Fig. 1B); the total protein expression level and the cell density were unchanged (data not shown). Consistent with this interpretation are the results of fluorescence microscopy. Cells expressing P39A tetra-Cys CRABP and treated with high salt show uniformly distributed fluorescence throughout the entire cell volume, consistent with the presence of soluble FlAsH-labeled protein, whereas cells expressing P39A tetra-Cys CRABP at low salt show dense hyperfluorescent regions near the poles of the cells (see Fig. 1A Lower).
Fig. 1.
Osmotic upshift suppresses protein aggregation. (A) (Upper) Time course of the bulk FlAsH fluorescence signal at 530 nm (excitation 500 nm) of E. coli BL21(DE3) cells expressing P39A tetra-Cys CRABP and subjected at the time of isopropyl-β-d-thiogalactoside induction to medium at differing osmolalities (from 100 to 300 mM NaCl). Note the behavior of the completely soluble tetra-Cys CRABP. (Lower) Examples of fluorescence microscopy images of the P39A tetra-Cys CRABP-expressing cells in low-salt medium (Left) and high-osmolality (+300 mM NaCl) medium (Right) taken 180 min after induction. (Magnification: ×1,000.) (B) Effects of the osmolality of the nutrient medium on the partitioning of P39A tetra-Cys CRABP between soluble and insoluble fractions. Note that tetra-Cys CRABP remains soluble at all sodium chloride concentrations (data not shown).
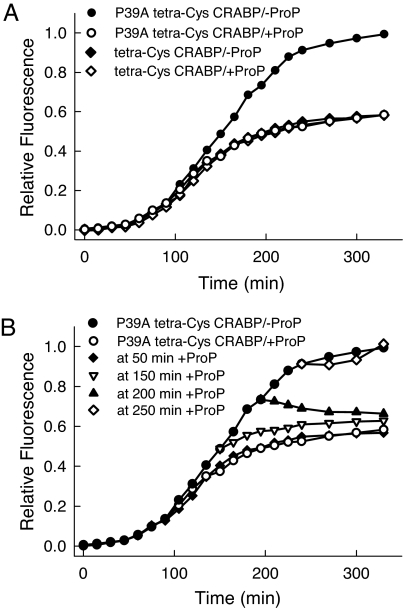
An osmotic shock, as initiated by the increased extracellular osmolality, is known to activate the ProP autotransporter in the cytoplasmic membrane, which actively takes up osmolytes from the extracellular medium (5, 29). The only osmolyte available in the medium was proline, which is transported by the ProP pump to an intracellular concentration >0.4 M (5). Any osmolyte readily available in the nutrient medium has priority over the synthesis of endogenous osmoprotectants (e.g., trehalose), which insures more rapid osmoadaptation of the cells (29). In this case, we have demonstrated that the contribution of endogenously synthesized osmolyte (most likely trehalose) to the solubilization of the P39A tetra-Cys CRABP is small and can be neglected by salt treatment in the absence of exogenous proline (Fig. 6, which is published as supporting information on the PNAS web site). To establish that increased intracellular concentrations of proline are responsible for the enhanced solubility of the aggregation-prone protein, we expressed both tetra-Cys CRABP and P39A tetra-Cys CRABP under conditions where proline uptake can be tightly controlled: in a ProP-deletion strain with an exogenous copy of ProP on a plasmid under control of the arabinose promoter (30). Inducing ProP by addition of arabinose simultaneous with induction of P39A tetra-Cys CRABP caused the same loss of the characteristic fluorescence enhancement signaling aggregate formation as was seen in the high-salt medium, whereas the aggregation behavior remained unchanged when no ProP was present (Fig. 2A). Strikingly, addition of arabinose to initiate biosynthesis of the ProP transporter (in the presence of salt to activate the ProP transporter) was effective up to 50 min after induction of synthesis of the aggregation-prone protein (before visible aggregates were formed), but not at later times (150 and 200 min after induction) (Fig. 2B). In the later phases of the aggregation process, proline appeared to prevent further growth of aggregates but was apparently not able to resolubilize completely the existing aggregates. At these later times, the bacteria have entered stationary phase, and the process dominating the FlAsH signal is growth of existing aggregates as opposed to new synthesis.
Fig. 2.
Proline internalized at early times in vivo inhibits aggregation. (A) Time evolution of the aggregation of P39A tetra-Cys CRABP monitored by the bulk FlAsH fluorescence signal of labeled cells, under depleted (−ProP) and up-regulated (+ProP) ProP transporter conditions in the E. coli strain WG710. Expression of the soluble tetra-Cys CRABP is not influenced by the amount of the ProP transporter; it remains the same in the absence of the ProP transporter (−ProP) and upon its overexpression (+ProP). (B) Proline influx induced at early times leads to soluble expression of P39A tetra-Cys CRABP. Proline uptake by the ProP transporter was initiated at different times after induction by addition of arabinose and 300 mM NaCl. The expression curves in the absence of ProP (−ProP) and in the presence of fully activated ProP (+ProP) E. coli WG710 are included for comparison.
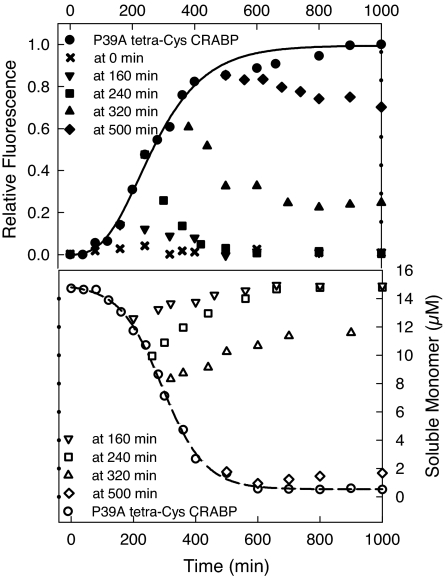
The effects of proline on aggregation, including its differential impact at early and late stages, could be fully recapitulated in vitro. Aggregation of P39A tetra-Cys CRABP I was initiated by dissolving the protein in a urea solution at a concentration that corresponds to the midpoint of a urea melting transition, as reported (27); under these conditions nucleation-polymerization aggregation kinetics were observed, with the kinetics consistent with a monomeric nucleus (27). When proline was added during the lag phase of aggregation or in early exponential aggregate growth (0–240 min), aggregation was inhibited fully (Fig. 3). Because high-intensity FlAsH fluorescence reports on unfolded species and is dominated by signal from aggregates, we attribute the drop of the fluorescence intensity to the solubilization and refolding of early aggregated species (Fig. 3). Adding proline at later times during the exponential phase of fluorescence increase (at 320 and 500 min) prevented further aggregate growth, but did not lead to solubilization of existing aggregates (which would have been indicated by a decrease to fluorescence characteristic of tetra-Cys CRABP); the soluble monomer concentration remained almost unchanged for the 500-min sample (Fig. 3). The addition of proline at 320 min led to a slight increase in the soluble monomer concentration, which suggests a partial solubilization of the existing aggregates. These in vitro results are remarkably parallel to the observations made in vivo.
Fig. 3.
Proline inhibits aggregation at early stages in vitro. The aggregation of 15 μM P39A tetra-Cys CRABP in vitro in the absence or presence of 500 mM proline added at different times is shown. The time evolution of the aggregation was monitored either by FlAsH fluorescence (Upper) or by determination of the quantity of soluble protein remaining after high-speed centrifugation (Lower).
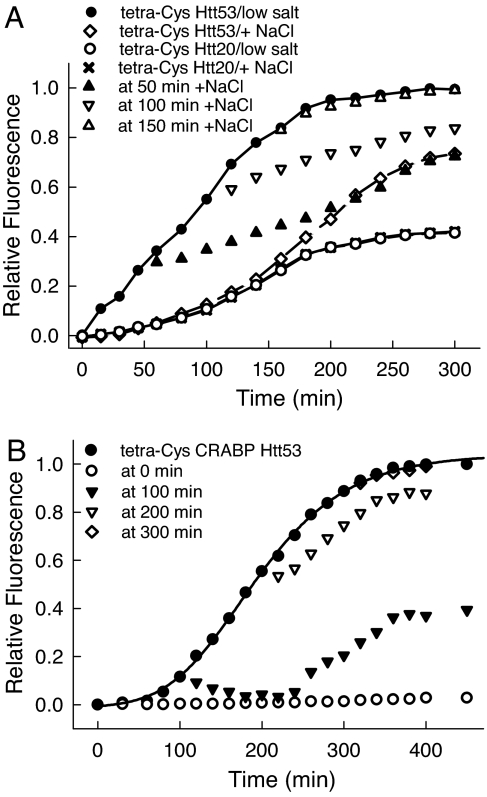
The generality of the modulation of aggregation by proline was tested by using C-terminal chimeras between tetra-Cys CRABP and exon 1 of Htt containing different polyQ lengths (n = 20 for the nonpathological and n = 53 for the pathological range). We have shown that the fusion construct with the longer polyQ tract aggregates rapidly both in vivo and in vitro via a mechanism that may involve structural perturbation of the adjacent tetra-Cys CRABP (28). Intriguingly, in vivo aggregates of tetra-Cys CRABP Htt53 were found to evolve over time, converting from a detergent-soluble state at early times to a detergent-insoluble state at later times. The aggregates at later times resemble the characteristic fibrillar amyloid-like aggregates that are hallmarks of polyQ pathologies. Interestingly, unlike the observations with P39A tetra-Cys CRABP, the osmotic up-shift and consequent increased intracellular proline concentration could not abolish the aggregation of tetra-Cys CRABP Htt53 completely (Fig. 4A). Instead, there was a change in the properties of isolated aggregates: The presence of proline in the cells at the time of induction of tetra-Cys CRABP Htt53 synthesis or in the early exponential phase of aggregate growth (up to 120 min) led to the accumulation of only SDS-soluble aggregates. As in the case of P39A tetra-Cys CRABP, proline uptake at later times in the aggregation process (>150 min after induction) was essentially without any effect on aggregation; intracellular accumulation of proline was not able to prevent the formation of detergent-resistant aggregates (unpublished work). In vitro, proline was able to suppress aggregation if present from the initiation of the aggregation. When added in the early phases of the aggregation process (up to 100 min), proline extended the lag phase, decreased the aggregation rate, and suppressed the conversion of the aggregates into detergent-resistant species (Fig. 4B). Addition at later stages of aggregation (200 and 300 min) had very little effect on aggregation.
Fig. 4.
Proline inhibits aggregation of a polyQ-containing chimera in vitro and in vivo. (A) Time evolution of the aggregation of tetra-Cys CRABP Htt53 and tetra-Cys CRABP Htt20 in E. coli BL21(DE3) cells cultured in medium of low salt or high osmolality (+300 mM NaCl). Note that the expression curves for tetra-Cys CRABP Htt20 in a low-salinity medium and with 300 mM NaCl-containing medium overlap and that the protein remains soluble over the entire expression cycle. (B) Proline (500 mM) was added to 5 μM aggregating tetra-Cys CRABP Htt53 in vitro at various time points after initiation of the aggregation reaction [by bringing the solution to 1 M urea (28)].
Discussion
These experiments establish that: (i) In vivo, proline disfavors aggregation of a slow-folding mutant of a globular protein (CRABP) and a chimera between CRABP and exon 1 of Htt (with the number of glutamines equal to 53). This effect of proline can be rationalized by relative destabilization of aggregation-prone monomeric intermediates, solubilization of small soluble-aggregated species, and stabilization and solubilization of the native state. (ii) When larger aggregates start to appear in the case of P39A tetra-Cys CRABP, proline impairs its further growth through addition of new monomers, but cannot reverse the aggregation process. (iii) Proline treatment when a polyQ-containing domain is expressed leads to formation of nonfibrillar, detergent-soluble inclusions in vivo. At later time points, proline does not block the formation of large aggregates, e.g., amyloidogenic fibrils.
We propose that proline is aggregation-protective because of its ability to suppress the earliest aberrant protein interactions that trigger pathogenic aggregation (Fig. 5). Our previous in vitro characterization of aggregation kinetics for both P39A tetra-Cys CRABP (27) and the tetra-Cys CRABP Htt53 chimera (28) indicated that they aggregate via a nucleation–polymerization mechanism with a monomeric nucleus. Recent studies of the physical chemical origins of osmolyte action (9) provide a formalism to account for our observations on modulation of aggregation by the osmolyte proline. Generally, aggregation propagates from partially folded or unfolded species that expose a greater backbone surface area than the native protein. As pointed out by Auton and Bolen (9), the unfavorable interactions of proline with the peptide backbone cause preferential exclusion of solute from the protein–water interface of this partially folded intermediate, thus increasing its free energy. Additionally, favorable interactions of proline with exposed side chains stabilize the native state (9), thus increasing the free-energy difference between the native and partially folded ensembles. In the early stages of aggregation, proline should favor the native state over the partially folded small aggregates in the same fashion as the partially folded monomer, because early aggregates will not sequester substantial backbone surface area (Fig. 5). We have measured the enhancement in native-state stability of P39A tetra-Cys CRABP in the presence of proline, both in vivo and in vitro, and it is entirely consistent with the expectation assuming that the differential solvent exposure of side chains and backbone groups accounts for the enhanced stability (9) (Fig. 7 and Table 1, which are published as supporting information on the PNAS web site). Thus, although we do not know the structure of the aggregation nucleus, we can safely assume that it will have greater exposed backbone surface area than the native state and thus be preferentially destabilized by proline. Late aggregates with high molecularity have a significantly smaller exposed surface, and thus the unfavorable osmolyte interactions would be relatively diminished. The earlier aggregates with low molecularity will be affected by proline to an extent that is determined by their exposed surface area. In our hands, the formation of these smaller aggregates is disfavored if proline is present throughout the aggregation process. These effects also account for our observations of proline’s influence on the aggregation processes of the chimera between the globular CRABP and Htt exon 1 at different points in the aggregation time course. Early, aggregation is disfavored, and later, after the transition from small amorphous (detergent soluble) to larger fibrillar (detergent insoluble) aggregates, proline is no longer able to block aggregation. More detailed study of the mechanism of inhibition of aggregation by this osmolyte is required.
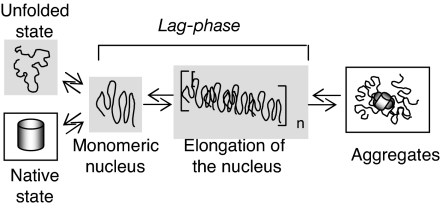
Fig. 5.
A schematic model of proline action on various species in the aggregation pathway. The disfavored species are shown with shadowing, and favored species are indicated by boxes. The solvophobic properties of proline disfavor species with greater solvent-exposed surface area, e.g., partially folded (nucleus) and unfolded ensembles. At early stages, intramolecular interactions are favored by the addition of proline, leading to a relative stabilization of the native state. In the later phases, intermolecular interactions are favored, promoting the formation of aggregated species that effectively sequester solvent-accessible surface area.
Promising results on modulation of aggregation by osmolytes including proline have been reported (10, 11, 16, 17). Trehalose was found to disfavor aggregation of the yeast prion protein Sup43 maintaining it in a partially folded state (23) and to confer a protective effect by reducing aggregation in a mouse model of oculopharyngeal muscular dystrophy (18). Another report (19) found that the natural osmolytes glycerol and trimethylamine N-oxide favored conversion of amorphous aggregates of amyloid Aβ into fibrillar aggregates. Recently, several researchers (17, 18) have reported aggregation inhibition and beneficial physiological effects (in mouse models) from trehalose treatment. Proline has previously been reported to disfavor aggregation in vitro (10, 11). However, in none of these cases is a molecular-level understanding of the phenomenon available. Moreover, the observed effects of proline seem particularly well suited to controlling the distribution of species in an aggregating system. The ability to control the composition of an aggregating system may help provide an improved understanding of the pathological effects of incompletely folded states, preaggregates, and large aggregates (31). Most excitingly, this improved understanding of molecular interactions behind aggregate formation should provide a basis for design of both diagnostic approaches and therapeutic strategies for misfolding and aggregation diseases. Additionally, those seeking optimal conditions for expression of a desired protein product in Escherichia coli may want to explore the ability of proline to diminish or abolish competing aggregation reactions.
Methods
In Vivo Assays.
P39A tetra-Cys CRABP and tetra-Cys CRABP (25) were subcloned into a pHSG398 vector (CmR; Takara Bio, Tokyo, Japan) behind a Ptac promoter and expressed in the E. coli hosts, BL21(DE3) or WG710 (32). Tetra-Cys CRABP Htt20 and tetra-Cys CRABP Htt53 fusions (exon 1 of Htt with various lengths of the polyQ stretch fused to the C terminus of tetra-Cys CRABP) were under the control of the T7 promoter and inserted in the pET16b vector as described (28). E. coli BL21(DE3) cells transformed with the tetra-Cys constructs and cultured at 37°C in glucose-containing minimal medium with low salt (30) were preloaded with FlAsH dye (25) one generation before induction of protein biosynthesis with 0.4 mM isopropyl-β-d-thiogalactoside and/or osmotic up-shift of the medium. FlAsH fluorescence of the bulk cell suspension at 37°C was monitored at 530 nm (excitation wavelength 500 nm; bandwidth 2 nm) (25). Fluorescence of cells treated identically but expressing CRABP without a tetra-Cys motif was used as a blank, and each point from the time-course curves was corrected by subtraction of the blank values. Protein fractionation into soluble and insoluble components was as described (25). Sterile NaCl solution was used to increase the osmolality of the nutrient medium. Simultaneous with the osmotic up-shift, the medium was supplied exogenously with 20 mM proline. The endogenous response of the cells (synthesis of any endogenous osmolytes, e.g., trehalose) to the increased salinity was tested by increasing the medium osmolality without providing exogenous proline. The strain E. coli WG710 is a ProP-deletion mutant, in which the function of the ProP transporter can be selectively restored by a stably transformed plasmid (pDC80) bearing the ProP gene behind an arabinose controllable promoter (32). Simultaneous with the induction of the biosynthesis of ProP with 0.2% arabinose, NaCl was supplied at 300 mM to maintain the active pump (30). The exogenous proline concentration was adjusted to 20 mM simultaneous with the osmotic up-shift and consequent ProP induction. Partitioning of the tetra-Cys variants between the soluble and insoluble fractions was determined by densitometry of Coomassie-stained gels after fractionation of the cells as described (25).
In Vitro Assays.
P39A tetra-Cys CRABP and tetra-Cys CRABP Htt53 were purified and labeled with FlAsH-EDT2 as described (27, 28), and the in vitro aggregation was monitored in the absence or presence of 0.5 M proline by two independent methods: quantifying the soluble protein after high-speed centrifugation or FlAsH fluorescence of the unfractionated solution as described (27, 28).
Fluorescence Microscopy.
Two microliters of FlAsH-labeled P39A tetra-Cys CRABP-expressing cells were withdrawn at different times after induction, immobilized on 1% agarose in LB medium, and imaged with a Nikon (Tokyo, Japan) Eclipse E600 fluorescence microscope (excitation at 485 nm and a 510-nm emission cut-off filter).
Supplementary Material
Acknowledgments
We thank Janet Wood (University of Guelph, Guelph, Canada) for providing E. coli strain WG710 and helpful discussions about osmotic stress response in E. coli, Matt Auton and Wayne Bolen for informative discussions regarding osmolyte functions, and Joanna Swain for critically reading the manuscript. This research was supported by National Institutes of Health Grant GM027616 (to L.M.G.) and Heisenberg Foundation Grant IG73/1-1 (to Z.I.).
Abbreviations
- CRABP
cellular retinoic acid-binding protein
- FlAsH
4′,5′-bis(1,3,2-dithioarsolan-2-yl)fluorescein
- Htt
huntingtin
- polyQ
polyglutamine.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
See Commentary on page 13265.
References
- 1.Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 2.Yancey P. H. J. Exp. Biol. 2005;208:2819–2830. doi: 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- 3.Record M. T., Jr., Courtenay E. S., Cayley D. S., Guttman H. J. Trends Biochem. Sci. 1998;23:143–148. doi: 10.1016/s0968-0004(98)01196-7. [DOI] [PubMed] [Google Scholar]
- 4.Bolen D. W., Baskakov I. V. J. Mol. Biol. 2001;310:955–963. doi: 10.1006/jmbi.2001.4819. [DOI] [PubMed] [Google Scholar]
- 5.Cayley S., Record M. T., Jr. Biochemistry. 2003;42:12596–12609. doi: 10.1021/bi0347297. [DOI] [PubMed] [Google Scholar]
- 6.Courtenay E. S., Capp M. W., Anderson C. F., Record M. T., Jr. Biochemistry. 2000;39:4455–4471. doi: 10.1021/bi992887l. [DOI] [PubMed] [Google Scholar]
- 7.Bolen D. W. Methods. 2004;34:312–322. doi: 10.1016/j.ymeth.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Samuel D., Kumar T. K., Jayaraman G., Yang P. W., Yu C. Biochem. Mol. Biol. Int. 1997;41:235–242. doi: 10.1080/15216549700201241. [DOI] [PubMed] [Google Scholar]
- 9.Auton M., Bolen D. W. Proc. Natl. Acad. Sci. USA. 2005;102:15065–15068. doi: 10.1073/pnas.0507053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel D., Kumar T. K., Ganesh G., Jayaraman G., Yang P. W., Chang M. M., Trivedi V. D., Wang S. L., Hwang K. C., Chang D. K., Yu C. Protein Sci. 2000;9:344–352. doi: 10.1110/ps.9.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar T. K., Samuel D., Jayaraman G., Srimathi T., Yu C. Biochem. Mol. Biol. Int. 1998;46:509–517. doi: 10.1080/15216549800204032. [DOI] [PubMed] [Google Scholar]
- 12.Stefani M., Dobson C. M. J. Mol. Med. 2003;81:678–699. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 13.Dobson C. M. Protein Pept. Lett. 2006;13:219–227. doi: 10.2174/092986606775338362. [DOI] [PubMed] [Google Scholar]
- 14.Koo E. H., Lansbury P. T., Jr., Kelly J. W. Proc. Natl. Acad. Sci. USA. 1999;96:9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross C. A., Poirier M. A. Nat. Med. 2004;10(Suppl.):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M., Machida Y., Nukina N. J. Mol. Med. 2005;83:343–352. doi: 10.1007/s00109-004-0632-2. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M., Machida Y., Niu S., Ikeda T., Jana N. R., Doi H., Kurosawa M., Nekooki M., Nukina N. Nat. Med. 2004;10:148–154. doi: 10.1038/nm985. [DOI] [PubMed] [Google Scholar]
- 18.Davies J. E., Sarkar S., Rubinsztein D. C. Hum. Mol. Genet. 2006;15:23–31. doi: 10.1093/hmg/ddi422. [DOI] [PubMed] [Google Scholar]
- 19.Yang D. S., Yip C. M., Huang T. H., Chakrabartty A., Fraser P. E. J. Biol. Chem. 1999;274:32970–32974. doi: 10.1074/jbc.274.46.32970. [DOI] [PubMed] [Google Scholar]
- 20.Fung J., Darabie A. A., McLaurin J. Biochem. Biophys. Res. Commun. 2005;328:1067–1072. doi: 10.1016/j.bbrc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 21.Liu R., Barkhordarian H., Emadi S., Park C. B., Sierks M. R. Neurobiol. Dis. 2005;20:74–81. doi: 10.1016/j.nbd.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Kanapathipillai M., Lentzen G., Sierks M., Park C. B. FEBS Lett. 2005;579:4775–4780. doi: 10.1016/j.febslet.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 23.Singer M. A., Lindquist S. Mol. Cell. 1998;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 24.Scaramozzino F., Peterson D. W., Farmer P., Gerig J. T., Graves D. J., Lew J. Biochemistry. 2006;45:3684–3691. doi: 10.1021/bi052167g. [DOI] [PubMed] [Google Scholar]
- 25.Ignatova Z., Gierasch L. M. Proc. Natl. Acad. Sci. USA. 2004;101:523–528. doi: 10.1073/pnas.0304533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams S. R., Campbell R. E., Gross L. A., Martin B. R., Walkup G. K., Yao Y., Llopis J., Tsien R. Y. J. Am. Chem. Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 27.Ignatova Z., Gierasch L. M. Biochemistry. 2005;44:7266–7274. doi: 10.1021/bi047404e. [DOI] [PubMed] [Google Scholar]
- 28.Ignatova Z., Gierasch L. M. J. Biol. Chem. 2006;281:12959–12967. doi: 10.1074/jbc.M511523200. [DOI] [PubMed] [Google Scholar]
- 29.Wood J. M. Microbiol. Mol. Biol. Rev. 1999;63:230–262. doi: 10.1128/mmbr.63.1.230-262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Racher K. I., Culham D. E., Wood J. M. Biochemistry. 2001;40:7324–7333. doi: 10.1021/bi002331u. [DOI] [PubMed] [Google Scholar]
- 31.Jahn T. R., Radford S. E. FEBS J. 2005;272:5962–5970. doi: 10.1111/j.1742-4658.2005.05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culham D. E., Henderson J., Crane R. A., Wood J. M. Biochemistry. 2003;42:410–420. doi: 10.1021/bi0264364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.