Abstract
Prions are novel kinds of hereditary units, relying solely on proteins, that are infectious and inherited in a non-Mendelian fashion. To date, they are either based on autocatalytic modification of a 3D conformation or on autocatalytic cleavage. Here, we provide further evidence that in the filamentous fungus Podospora anserina, a MAP kinase cascade is probably able to self-activate and generate C, a hereditary unit that bears many similarities to prions and triggers cell degeneration. We show that in addition to the MAPKKK gene, both the MAPKK and MAPK genes are necessary for the propagation of C, and that overexpression of MAPK as that of MAPKKK facilitates the appearance of C. We also show that a correlation exists between the presence of C and localization of the MAPK inside nuclei. These data emphasize the resemblance between prions and a self-positively regulated cascade in terms of their transmission. This thus further expands the concept of protein-base inheritance to regulatory networks that have the ability to self-activate.
Keywords: epigenetic, prion, Podospora anserina
In addition to prions based on hereditary transmission of alternative 3D conformation of cellular proteins (1), Roberts and Wickner (2) recently described in the yeast Saccharomyces cerevisiae a novel kind of hereditary unit that propagates by proteolytic cleavage of a PrB pro-protease by the corresponding mature PrB protease. Because the behavior of the mature protease bears resemblance to prions, the mature protein was designated [β] prion. Indeed, [β] is infectious and can be reversibly cured; its generation depends on the PrB gene and is enhanced when the pro-protease is overexpressed. It was also proposed that many enzymes may display a similar property and thus create phenomena akin to those triggered by prions based on conformational changes in protein structure.
Crippled Growth (CG) corresponds to an epigenetic cell degeneration phenomenon of the filamentous fungus Podospora anserina caused by C, a hereditary unit that resembles the [β] prion by the way it manifests itself (3). Cell degeneration is easily visible macroscopically and appears as sectors of poor growth with a pigmented, flat, and female-sterile mycelium (CG growth) as opposed to normal growth (NG growth). As is the case of yeast and fungal prions, C is cytoplasmic and infectious. It can also be reversibly cured by stress. However, unlike other prions, C requires special conditions to propagate. It cannot propagate in wild-type growing hyphae on standard M2 medium, whereas it propagates and creates CG in wild-type cultures grown on M2 medium supplemented with yeast extract (4). C can propagate in PDC mutants (with mutations that Promote the Development of CG). Indeed, these mutants develop CG in growth conditions for which wild-type cultures never display CG, e.g., on medium lacking yeast extract (4). To date, the only genes affected in PDC mutants and identified at the molecular level are genes that control translation accuracy (3, 4). Another unique property exhibited by C is that C can be induced by a physiological stimulus with 100% efficiency, i.e., by allowing the cultures to enter stationary phase (3). Hence, C is present in CG cultures both during growth and in stationary phase, but it is also present in all NG cultures specifically during the stationary phase even though the cultures appear normal. Upon growth renewal, C is eliminated from the growing hyphae yielding a NG mycelium, except in defined conditions that permit wild-type cultures to present CG or in the PDC mutants.
A mutational analysis made it possible to recover genes necessary for the development of CG, the IDC genes (4–6). Some of these genes have now been cloned. Their identification led to the following observations. First, PaASK1, a MAP kinase kinase kinase (MAPKKK), is necessary for C production, and when PaASK1 is overexpressed, C propagation is facilitated, i.e., wild-type cultures carrying overexpressed PaASK1 display CG (5). Prion proteins exhibit these properties (7). Second, the PaNox1 NADPH oxidase is also required for C production, and based on epistasis analysis, it most likely acts upstream of PaASK1 (6). It is noteworthy that, unlike what is observed with conformation-based prions, the inactivation of either gene does not yield the same phenotype as when C is present (7).
Because of the similarities and differences between C and prions, we proposed a related model (5) taking into account that the MAPKKK is at the top of a succession of three kinases (the second and third being the MAP kinase kinase or MAPKK and the MAP kinase or MAPK). We propose that PaASK1 might not directly self-activate as proposed for the PrB protease, but that the entire MAPK cascade consisting of the MAPKKK, MAPKK, and MAPK proteins, but also possibly including other members of the cascade such as PaNox1, may self-activate. Under this assumption, C would be the active state of the entire cascade and would replicate by activating in trans other nonactive cascade components present in the cells. Because P. anserina as all filamentous fungi presents a coenocytic structure, C could also spread to neighboring cells and trigger CG. Here, we provide further evidence that the active MAPK module could indeed be part of the C hereditary unit.
Results
Cloning and Inactivation of the MAPKK and MAPK Genes Acting Downstream of PaASK1.
The PaASK1 MAPKKK is the orthologue of the S. cerevisiae Bck1 gene that encodes the MAPKKK of the cell integrity pathway (5). We cloned the P. anserina orthologues of the S. cerevisiae MKK1/MKK2 and Mpk1 (= Slt2) genes that encode MAPKK and MAPK acting downstream of Bck1p, respectively (see Materials and Methods). A search of the P. anserina complete genome sequence revealed that a single orthologue of each gene is present. Both MAPK and MAPKK contain the double phosphorylation consensus sequences expected for their activity. They are designated PaMKK1 for the MAPKK encoding gene and PaMpk1 for the MAPK encoding gene. PaMKK1 displays 46% identity and 60% similarity with the MKK1p over 370 aa, and PaMpk1 displays 68% identity and 81% similarity with the Slt2p over 360 aa.
The PaASK1 null mutants were obtained by UV mutagenesis (5), and several unassigned mutants with the same sterility and depigmented phenotypes as the PaASK1 mutant were retrieved in the same screen; they could correspond to either PaMKK1 or PaMpk1 (4). Plasmids containing the complete PaMKK1 and PaMpk1 genes were constructed and used to transform the candidate mutants. The PaMpk1-carrying plasmid did not complement any mutant, whereas the PaMKK1-carrying plasmid complemented the IDC404 and IDC505 mutants that belong to the same complementation and recombination group. Sequencing PaMKK1 in the two mutant strains confirmed that these two mutants had an inactive PaMKK1 allele because the CAA codon 237 was mutated to a TAA stop codon in IDC404 and the TAT codon 415 was also mutated to TAA in IDC505. Hence, in both mutants, part of the catalytic domain of the kinase is missing. Because no mutant for PaMpk1 was retrieved, the PaMpk1 coding sequence was replaced by a phleomycin resistance marker through a single-step gene replacement (see Materials and Methods) to yield the ΔPaMpk1 mutant. Finally, to test the mutants for CG, the IDC404 AS6-5, IDC505 AS6-5 and ΔPaMpk1 AS6-5 strains were produced by crossing with the AS6-5 mutant. AS6-5 is a mutation that permits the development of a strong CG phenotype (3). The IDC404, IDC505, and ΔPaMpk1 mutants exhibited the same phenotype as the PaASK1 mutants, including the inability to present CG after passage into stationary phase (5). Similar to loss of PaASK1, this inability is due to the incapacity of the mutants to produce and sustain C. First, the IDC404 AS6-5, IDC505 AS6-5, and ΔPaMpk1 AS6-5 strains never present CG when they exit stationary phase. Second, C donors in cell fusion experiments do not infect these mutants (Table 1). Third, unlike the wild-type strain, the mutants cannot infect the AS6-5 strain, even if they are previously infected with an AS6-5 CG C donor strain (Table 1). The IDC404 and IDC505 strains were transformed with the wild-type PaMKK1 allele, and the ΔPaMpk1 strain was transformed with the wild-type PaMpk1 allele. The transformants recovered behaved as wild-type, confirming that the phenotypes observed were due to inactivation of PaMKK1 or PaMpk1.
Table 1.
PaMKK1 and PaMpk1 are required for production of C
| Donor strains | Recipient strains | C transmission |
|---|---|---|
| CG AS6-5* | NG AS6-5 | 30/30 |
| CG AS6-5* | AS6-5 IDC | 0/30 |
| CG AS6-5* | AS6-5 IDC | 0/30 |
| CG AS6-5* | AS6-5 IDC | 0/30 |
| CG AS6-5* | AS6-5ΔPaMpk1 | 0/30 |
| WT† | NG AS6-5 | 30/30 |
| IDC† | NG AS6-5 | 0/30 |
| IDC† | NG AS6-5 | 0/30 |
| IDC† | NG AS6-5 | 0/30 |
| ΔPaMpk1† | NG AS6-5 | 0/30 |
| IDC‡ | NG AS6-5 | 0/30 |
| IDC‡ | NG AS6-5 | 0/30 |
| ΔPaMpk1‡ | NG AS6-5 | 0/30 |
Shown are the number of successful contaminations of the indicated recipient strains by explants taken from the indicated donor strains (C donor) in experimental conditions identical to those of ref. 5. Cell fusion at the junction between recipient and donor is a natural phenomenon in P. anserina. With this system, up to 100% transmission is achieved in control experiments. None were observed in the PaMKK1 and PaMpk1 mutants.
*Explants were taken from the growing margin of the CG AS6-5 culture.
†Explants were taken from the stationary phase area of the indicated cultures, i.e., at the center of the thallus.
‡Explants were taken from cultures that were previously infected by a CG AS6-5 culture.
To prove that PaMpk1 acts downstream of PaASK1 and PaMKK1, its phosphorylation was measured in the wild-type strain and in the PaASK1 and PaMKK1 mutants. As seen in Fig. 1, PaMpk1 was detected in the wild-type strain in its phosphorylated and unphosphorylated states at 24, 36, and 48 h of growth. As expected, no PaMpk1 was detected in the ΔPaMpk1 strain. In the IDC118 and IDC404 mutants, the unphosphorylated form was readily detected, whereas the phosphorylated form was absent, showing that PaMKK1 and PaASK1 act upstream of PaMpk1.
Fig. 1.
PaMKK1 and PaASK1 act upstream of PaMpk1. Protein extracts (20 μg) from a wild-type strain grown for different durations (24, 36, and 48 h) on M2 medium and from the ΔPaMpk1, IDC404, and IDC118 mutants grown for 48 h were separated on gels and probed with antibodies that specifically detect the unphosphorylated form (anti-MAPK) and the phosphorylated form (anti-phospho-MAPK) of PaMpk1.
Overexpression of PaMpk1 Enhances CG.
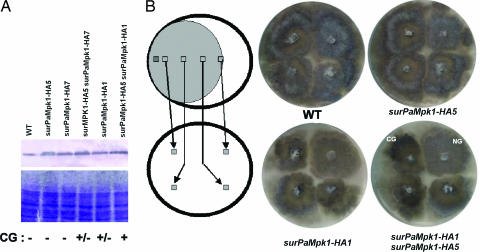
As described above, both PaMKK1 and PaMpk1, like PaASK1, are necessary for C production. To determine whether PaMpk1 can trigger the appearance of CG on M2 medium in an otherwise wild-type strain as observed for the PaASK1overexpression, it was overexpressed. To this end, PaMpk1 was tagged with HA and placed under the control of the gpd and AS4 promoters. gpd codes for glyceraldehyde-3 phosphate dehydrogenase (8), and AS4 codes for the eEF1A translation elongation factor (9). Both genes are strongly expressed throughout the life cycle of P. anserina. The resulting chimeras were introduced into P. anserina by transformation, and three independent transformants were selected for further studies. Two of them, surPaMpk1-HA5 and surPaMpk1-HA7, contain the PaMpk1-HA coding sequence driven by gpd, whereas surPaMpk1-HA1 is driven by AS4. A strain containing surPaMpk1-HA5 and surPaMpk1-HA7 and a strain containing surPaMpk1-HA5 and surPaMpk1-HA1 were constructed to further increase the amount of PaMpk1 present in the cells. Overexpression was monitored by Western blots. The amounts of PaMpk1-HA in the overexpressed strains were increased in various proportions, when compared with a strain containing the PaMpk1-HA coding sequence driven from the PaMpk1 promoter (Fig. 2A). The strains that overexpress the highest levels of PaMpk1-HA presented CG alterations in conditions where the wild-type strain did not (Fig. 2B). Cell fusion experiments showed that these strains contained C. This finding indicates that overexpression of PaMpk1 enhances CG as described for the overexpression of PaASK1. However, only the strongest overexpression of PaMpk1-HA promoted a stable CG, whereas the other overexpressing strains presented no or an unstable CG (Fig. 2B).
Fig. 2.
Overexpression of PaMpk1 enhances CG. (A) Quantification of the overexpression of PaMpk1-HA in the strains indicated. (Upper) Western blotting with anti-HA antibodies. (Lower) Coomassie staining of a gel with the same samples. The fold overexpression compared with wild-type (WT) has been evaluated on four different blots, of which the one presented in the figure is revealed by nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. In the three others, the HA-tag was detected with ECL. The values obtained after normalization with the wild-type strain were 1.7 ± 0.3 for surPaMpk1-HA5, 1.5 ± 0.1 for surPaMpk1HA7, 1.7 ± 0.5 for surPaMpk1-HA5 surPaMpk1HA7, 1.4 ± 0.5 for surPaMpk1-HA1, and 3.75 ± 1.0 for surPaMpk1-HA1 surPaMpk1-HA5. CG, ability of the strain to trigger CG. (B) Experimental setup to detect CG and resulting plates. CG is visible in the surPaMpk1-HA1 + surPaMpk1-HA5 overexpressing strain yielding darkly pigmented thalli and is less pronounced in the surPaMpk1-HA1 strain and in the surPaMpk1-HA5 + surPaMpk1-HA7 strain (not shown).
GFP-Tagging of PaMpk1.
Phenotypic analyses showed that C is present in wild-type strains during the stationary phase and yet it is not present in apical hyphae (3), but by using the phosphorylated-specific and unphosphorylated-specific antibodies, no clear difference was detected in the PaMpk1 phosphorylation status between these two growth stages (Fig. 1). We thus constructed a GFP-tagged version of PaMpk1 (see Materials and Methods) to monitor the in vivo behavior of PaMpk1. The resulting chimeric PaMpk1-GFP protein was functional because it complemented the defect of the ΔPaMpk1 mutant. However, pigmentation and fertility were slightly delayed in the complemented strain and interestingly displayed a weak CG, suggesting that PaMpk1-GFP was not as efficient as wild-type PaMpk1. No difference was observed in the localization of PaMpk1-GFP in strains carrying a wild-type copy of PaMpk1 or the deleted ΔPaMpk1 allele at the normal chromosomal locus.
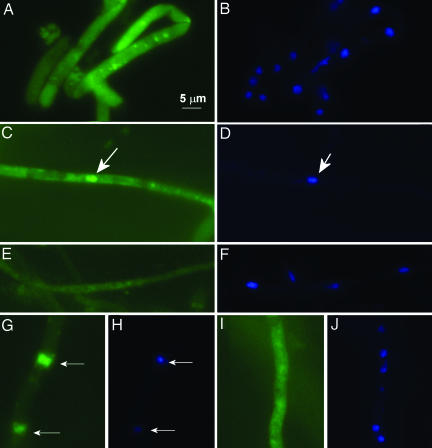
PaMpk1-GFP localization was confirmed in growing hyphae and in 3-day-old hyphae grown on M2 medium (Fig. 3). In the wild-type strain, PaMpk1-GFP presented a diffuse cytoplasmic localization in growing hyphae and very rarely accumulated inside nuclei (<1% of the nuclei were stained). Upon entrance into stationary phase, some PaMpk1-GFP entered nuclei and was accumulated in ≈20% of them. This nuclear localization typical of stationary phase was not seen in the IDC118 and IDC404 mutants. In NG AS6-5 cultures, PaMpk1-GFP displayed the same localization as in wild-type cultures. However, in the NG AS6-5 strain, ≈50% of the nuclei were stained in the stationary phase. Finally, in CG AS6-5 cultures, PaMpk1-GFP was present in ≈80% of the nuclei in both growing and stationary-phase hyphae.
Fig. 3.
Localization of PaMpk1-GFP. (A and B) Diffuse localization of PaMpk1-GFP in growing hyphae of a wild-type strain carrying the MGFP4 transgene. (C and D) Typical PaMpk1-GFP nuclear localization in 3-day-old hyphae of a wild-type strain with the MGFP4 transgene. (E and F) Lack of PaMpk1-GFP nuclear localization in 3-day-old hyphae in strains carrying the IDC404 mutation and the MGFP4 transgene. The same result is seen in strains with the IDC118 mutation carrying the MGFP4 transgene. (G and H) Nuclear localization of PaMpk1-GFP in growing hyphae of a CG AS6-5 culture carrying the MGFP4 transgene. (I and J) Diffuse localization of PaMpk1-GFP in growing hyphae of an NG AS6-5 culture carrying the MGFP4 transgene. A, C, E, G, and I: GFP staining to show PaMpk1-GFP. B, D, F, H, and J: DAPI staining to show nuclei. Arrows point toward nuclei containing PaMpk1.
Behavior of PaMpk1 in the IDC343 PaNox1 Mutant.
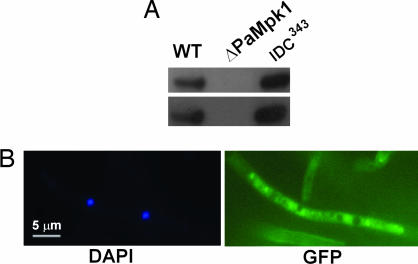
A mutant in the PaNox1 gene has been shown to display a phenotype similar to ΔPaMpk1, although less severe (6). We thus checked whether PaNox1 is necessary for PaMpk1 phosphorylation. Surprisingly, PaMpk1 was phosphorylated in the IDC343 PaNox1 mutant (Fig. 4A). However, the PaMpk1-GFP protein was never localized in the nuclei of the IDC343 PaNox1 mutant (Fig. 4B).
Fig. 4.
Effect of the PaNox1 mutation on PaMpk1. (A) Protein extract of the IDC343 mutant analyzed as in Fig. 1 shows that PaMpk1 is phosphorylated in a strain lacking PaNox1. (B) Lack of nuclear localization of PaMpk1 in 3-day-old hyphae.
Discussion
In previous investigations (5), we implicated the PaASK1 MAPKKK in the generation of the non-Mendelian C element and postulated a model based on self-sustained activation (hysteresis) of the PaASK1 cascade to explain the properties of C. In this model, C corresponds to the active state of the cascade. Here, this model is confirmed by demonstrating that the downstream MAPKK and MAPK are also required for the generation of C. In addition, overexpression of the PaMpk1 gene enhances CG. Like PaASK1, the PaMpk1 protein obeys the rules that would classify it as a prion, as defined previously (7), and it is likely that PaMKK1 has the same properties. The infectious unit would thus encompass at least the three kinases and possibly the PaNox1 NADPH oxidase because this latter enzyme is necessary to produce C (6).
We also show that a correlation exists between the nuclear localization of PaMpk1 and the presence of the C element. First, PaMpk1 is present during growth in a diffuse cytosolic form and enters certain nuclei upon entrance into the stationary phase in wild-type strains. This result is consistent with infection experiments showing that C is present in wild-type cultures during stationary phase (3). Such nuclear PaMpk1 is absent from the PaASK1 and PaMKK1 mutants that are unable to sustain C. Second, in the AS6-5 strain that can develop CG, a constitutive nuclear localization of PaMpk1 is detected in CG cultures, whereas in NG cultures, PaMpk1 is located inside the nucleus only after 2 days of growth as for the wild-type strain. In addition, more nuclei contain PaMpk1 in this strain, which tends to display CG. Third, a strain lacking the PaNox1 NADPH oxidase and unable to produce C during stationary phase does not exhibit the PaMpk1 nuclear localization. It is known that nuclear localization is involved in gene activation by MAP kinases (10) and that the Mpk1 protein from S. cerevisiae, which is orthologous to PaMpk1, is predominantly in the nucleus (11). Interestingly, PaMpk1 is phosphorylated in the PaNox1 mutant strain, indicating that, although it is not required for its phosphorylation, PaNox1 acts upstream of PaMpk1 to ensure its correct nuclear localization. In addition, phosphorylation of PaMpk1 is likely insufficient for activity, which also seems to require correct nuclear localization, because the PaNox1 mutant displays a phenotype very similar to ΔPaMpk1.
Together, these data support the model, wherein one or several self-activated positive loop(s) in the cascade ensure(s) a sustained activation, or hysteresis, that is sufficiently strong for the active MAPK module to be transmitted through mitosis and to neighboring cells during cell fusions (5). This loop creates a cytoplasmic and infectious factor permitting nuclear localization of PaMpk1 and that bears many similarities to prions. However, unlike prions, this protein-base genetic element would be made of several enzymes. Because a related MAPK cascade with a similar property has been described as being involved in the maturation of Xenopus eggs (12), hysteresis could be a general property of the MAPK pathways. In normal conditions, the cascade is activated upon entrance into stationary phase, leading to differentiation including erection of hyphae out of the medium and pigment accumulation that culminate with sexual reproduction. If this cascade is misactivated during growth, CG cell degeneration ensues. Deletion analyses of the PaASK1 gene suggest that hysteresis is dispensable for aerial hyphae and pigment accumulation but seems necessary for sexual reproduction (5). Further studies may aim at identifying which components of the cascade directly participate in hysteresis. This will require a better understanding of how the PaASK1 cascade is involved in the physiology of P. anserina and can be achieved by identifying the other factors that participate directly or indirectly in cascade activation and repression (4).
Materials and Methods
Strains and Culture Conditions.
The P. anserina strains used here were all derived from the S strain, ensuring a homogenous genetic background (13). The AS4-43 and AS6-5 strains possess the PDC mutations permissive for CG that are routinely used to test for the presence and effect of C (3, 5). The null alleles of PaASK1 (IDC118) and PaNox1 (IDC343) are described in refs. 5 and 6. Detailed standard culture conditions, media methods, and genetic methods for this fungal model system are available (14) and can be obtained at http://podospora.igmors.u-psud.fr.
Cloning of PaMpk1 and PaMKK1.
Cloning of the PaMpk1 gene was initiated before the release of the complete P. anserina genome sequence (accessible at http://podospora.igmors.u-psud.fr). Part of the PaMpk1 gene was amplified by PCR with oligonucleotides 5′-AARGARCTCGGNCARGGNGCNTAYGGNATHGT-3′ and 5′-TTRCGNCTRACRCTRGAGTTYTADACRCTRAA-3′ or oligonucleotides 5′-AARGARCTCGGNCARGGNGCNTAYGGNATHGT-3′ and 5′-AARTARATRGTYTARGARACRCC-3′ that correspond to conserved regions of the protein. The resulting PCR products were used to probe a cosmid bank, and two overlapping clones were retrieved and sequenced yielding the complete PaMpk1 sequence. The PaMKK1 gene was retrieved by an in silico search of the first 3X release of the complete genome sequence. Because the sequence was incomplete, a cosmid clone containing the PaMKK1 gene was screened by PCR with oligonucleotides 5′-TCAGTCTCCAATCCGCCAGC-3′ and 5′-CCTCCAGCCTTCATCGTCCA-3′ that specifically amplify PaMKK1. The PaMKK1 gene present in this clone was used to complete the sequence. GenBank accession numbers are AY452723 for PaMKK1 and AY452722 for PaMpk1. These sequences are identical to the ones obtained by the complete 10X genome project.
Deletion and Overexpression of the PaMpk1 Gene.
To delete PaMpk1, a BglII fragment containing the gene was circularized with ligase and used as substrate for PCR amplification with oligonucleotides 5′-AGCGGCCGCTATGGTCTGTTGGCTGCATTC-3′ and 5′-AGGGCCCTGGAGATCGCCCATGAT-3′. The amplified product was cloned into pGEMT (Promega, Madison, WI). The insert of one of the clones retrieved was cleaved with NotI and ligated into pBC-hygro linearized with NotI (15). One of the clones obtained was digested with BglII and used to transform the P. anserina S strain. This linearized plasmid could replace the first three exons of the PaMpk1 coding sequence by a hygromycin B resistance marker through homologous recombination. Hygromycin B resistant transformants were selected. Several transformants displayed a phenotype identical to the PaASK1 mutants. Southern blot analyses demonstrated that the marker correctly replaced PaMpk1. One transformant designated ΔPaMpk1 was crossed with a wild-type strain to purify the mutant and obtain it in association with the two mating types.
To overexpress the PaMpk1 protein, the PaMpk1 gene was amplified with primers 5′-CGCGCTCTAGACCCACACTCGGCCTCCGTCTTTTG-3′ and 5′-CGCGCAAGCTTTCTGCCTCCGAGCTCAGCCTCG-3′. This latter primer eliminates the PaMpk1 stop codon and introduces a HindIII restriction site. The PCR product was cloned into the pAKS106 vector digested with XbaI and HindIII. pASK106 derived from pBC-phleo (15) contains an HA tag sequence at the ClaI site followed by the ribP2 terminator. The HA-tag is in frame with the restriction sites from the polylinker. The insert of one of the resulting plasmids designated pAKS105 was sequenced. The PaMpk1 coding sequence contained no mutation and was in frame with the HA-tag at its C terminus. pAKS105 was used to transform the ΔPaMpk1 mutant. Numerous transformants with a wild-type phenotype were obtained, showing that the tagged gene was fully capable of complementing the PaMpk1 deletion. The gpd and AS4 promoters were then amplified from pBCH-gpd (see below) and pBC-HA (5), respectively, with primers 5′-CTAAAGGGAACAAAAGCTG-3′ and 5′-ACTATAGGGCGAATTGG-3′ and cloned into pGEMT. pBCH-gpd was constructed by inserting the EcoRI–NcoI fragment of pRP81-1 containing the gpd promoter (8) into the SmaI site of pBC-hygro. Upon cloning into pGEMT, both inserts could be easily excised with XbaI and NotI and cloned into pAKS105, resulting in plasmids in which the PaMpk1-HA coding sequence is under the control of the gpd or AS4 promoters. The resulting plasmids were used to transform the ΔPaMpk1 mutant, and numerous complementing transformants were obtained. Two transformants carrying the PaMpk1-HA coding sequence driven by gpd, surPaMpk1-HA5, surPaMpk1-HA7, and one driven by AS4, surPaMpk1-HA1, were selected for further studies.
Protein Extracts and Western Blot Analysis.
After growing for various lengths of time, P. anserina mycelia were harvested in 1.5-ml tubes, resuspended in 1 vol of 20 mM Tris·HCl (pH 7.5), containing 100 mM NaCl and 1 mM EDTA supplemented with 0.5% Triton X-100, and disrupted by fragmentation in a FastPrep apparatus (Qbiogene, Irvine, CA) set at position 5 for 2.5 min in the presence of 30 μl of glass beads (diameter, 0.25–0.5 mm). The lysate was centrifuged at 4°C for 5 min at 4,000 × g. The protein concentration in the supernatant was estimated by the Bio-Rad (Hercules, CA) protein assay, with BSA as standard. Crude protein extracts (10 μg) were separated by SDS/PAGE, using an 8% polyacrylamide gel (16). The proteins separated in the gel were detected by direct staining with Coomassie brilliant blue or immunodetected after semidry transfer to PVDF membranes (Hybond-P; GE Healthcare, Piscataway, NJ) at 180 mA for 1 h. After transfer, the membranes were soaked in TBS-T (20 mM Tris·HCl, pH 7.5/150 mM NaCl/0.05% Tween 20) containing 5% (wt/vol) lowfat milk.
To detect the bands corresponding to MAPK-tagged HA proteins, a specific rat monoclonal anti-HA antibody (3F10; Roche Applied Science, Indianapolis, IN) was used. This antibody recognizes no protein in extracts from wild-type or ΔPaMpk1 P. anserina. The membranes were incubated for 1 h at 37°C with this antibody diluted 2,500-fold in TBS-T. After thorough rinsing with 1% nonfat milk in TBS-T, the membranes were incubated for 1 h with a goat anti-rat total Ig (H + L) antibody conjugated to alkaline phosphatase (Promega). The specific bands were then revealed by the nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate test (Promega) or by the ECL test (GE Healthcare). The p44/p42 MAP kinase antibody and the phospho-p44/p42 MAP kinase antibody from Cell Signaling Technology (Danvers, MA) were used as recommended by the supplier to detect unphosphorylated and phosphorylated PaMpk1, respectively.
PaMpk1 GFP-Tagging.
To tag PaMpk1 with the GFP, plasmid pAKS105 was cleaved with XbaI and HindIII, and the 1.6-kb fragment containing PaMpk1 lacking its stop codon was purified. The GFP coding sequence was excised from plasmid pEGFP-1 (Clontech, Mountain View, CA) with HindIII and NotI, and the corresponding fragment was purified. Both the PaMpk1 and GFP coding sequences were ligated into pBC-hygro digested with XbaI and NotI to yield plasmid pMGFP1. The plasmid was introduced into the wild-type strains by transformation, and six transformants were crossed with ΔPaMpk1. Two independent transgenes (MGP1 and MGFP4) capable of complementing the phenotypes of the deletion were selected for further studies and re-associated by genetic crosses with AS6-5, IDC404, IDC118, or IDC343. Both transgenes yielded the same results.
Pictures were taken with a Leica (Bannockburn, IL) DMIRE 2 microscope and analyzed with ImageJ (http://rsb.info.nih.gov/ij).
Acknowledgments
We thank Fabienne Malagnac for useful discussions and critical reading of the manuscript, Marc-Henri Lebrun for providing antibodies, Magali Prigent for technical expertise, and Anne-Lise Haenni for correcting the manuscript. S.K. is the recipient of a fellowship from the Ministère de l’Education et de la Recherche. This work was supported by the Action Concertée Incitative “Encéphalopathies Spongiformes Subaiguës Transmissibles (ESST) et Prions” (Reference 2000-37) and funding from the Centre National de la Recherche Scientifique/Université de Paris-Sud 11, and was performed in compliance with the current laws governing genetic experimentation in France.
Footnotes
References
- 1.Wickner R. B., Edskes H. K., Roberts B. T., Baxa U., Pierce M. M., Ross E. D., Brachmann A. Genes Dev. 2004;18:470–485. doi: 10.1101/gad.1177104. [DOI] [PubMed] [Google Scholar]
- 2.Roberts B. T., Wickner R. B. Genes Dev. 2003;17:2083–2087. doi: 10.1101/gad.1115803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silar P., Haedens V., Rossignol M., Lalucque H. Genetics. 1999;151:87–95. doi: 10.1093/genetics/151.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haedens V., Malagnac F., Silar P. Fungal Genet. Biol. 2005;42:564–577. doi: 10.1016/j.fgb.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Kicka S., Silar P. Genetics. 2004;166:1241–1252. doi: 10.1534/genetics.166.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malagnac F., Lalucque H., Lepère G., Silar P. Fungal Genet. Biol. 2004;41:982–997. doi: 10.1016/j.fgb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Baxa U., Taylor K. L., Steven A. C., Wickner R. B. Contrib. Microbiol. 2004;11:50–71. doi: 10.1159/000077062. [DOI] [PubMed] [Google Scholar]
- 8.Ridder R., Osiewacz H. D. Curr. Genet. 1992;21:207–213. doi: 10.1007/BF00336843. [DOI] [PubMed] [Google Scholar]
- 9.Silar P., Picard M. J. Mol. Biol. 1994;235:231–236. doi: 10.1016/s0022-2836(05)80029-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen R. H., Sarnecki C., Blenis J. Mol. Cell. Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Drogen F., Peter M. Curr. Biol. 2002;12:1698–1703. doi: 10.1016/s0960-9822(02)01186-7. [DOI] [PubMed] [Google Scholar]
- 12.Bagowski C. P., Ferrell J. E., Jr. Curr. Biol. 2001;11:1176–1182. doi: 10.1016/s0960-9822(01)00330-x. [DOI] [PubMed] [Google Scholar]
- 13.Rizet G. Rev. Cytol. Biol. Veg. 1952;13:51–92. [Google Scholar]
- 14.Esser K. In: Handbook of Genetics. King R. C., editor. Vol. 1. New York: Plenum; 1974. pp. 531–551. [Google Scholar]
- 15.Silar P. Fungal Genet. Newsletter. 1995;42:73. [Google Scholar]
- 16.Laemmli U. K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]