Abstract
Glucagon-like peptide 1 (GLP-1) is a hormone that has received significant attention as a therapy for diabetes because of its ability to stimulate insulin biosynthesis and release and to promote growth and survival of insulin-producing β cells. While GLP-1 is produced from the proglucagon precursor by means of prohormone convertase (PC) 1/3 activity in enteroendocrine L cells, the same precursor is differentially processed by PC2 in pancreatic islet α cells to release glucagon, leaving GLP-1 trapped within a larger fragment with no known function. We hypothesized that we could induce GLP-1 production directly within pancreatic islets by means of delivery of PC1/3 and, further, that this intervention would improve the viability and function of islets. Here, we show that adenovirus-mediated expression of PC1/3 in α cells increases islet GLP-1 secretion, resulting in improved glucose-stimulated insulin secretion and enhanced survival in response to cytokine treatment. PC1/3 expression in α cells also improved performance after islet transplantation in a mouse model of type 1 diabetes, possibly by enhancing nuclear Pdx1 and insulin content of islet β cells. These results demonstrate a unique strategy for liberating GLP-1 from directly within the target organ and highlight the potential for up-regulating islet GLP-1 production as a means of treating diabetes.
Keywords: diabetes, islet transplantation, prohormone convertase 1/3
Type 1 diabetes (T1D) is an autoimmune disease in which insulin-producing pancreatic β cells are destroyed, rendering affected individuals dependent on exogenous insulin to control blood glucose levels. A cure for T1D will likely require replacement of lost β cell function by means of transplantation of islets or “surrogate” insulin-producing cells or induction of endogenous β cell regeneration to restore a functional mass of β cells. Although transplantation of pancreatic islets can be effective in T1D patients, broad implementation of this therapy is limited by the large amount of tissue required per patient (1, 2). This requirement may in part be due to poor survival of transplanted islets in the first few days after transplantation, perhaps because of glucotoxicity, hypoxia, and/or inflammation (3, 4).
Glucagon-like peptide 1 (GLP-1) is a hormone with multiple activities that help maintain blood glucose homeostasis (5). It enhances insulin secretion in a glucose-dependent manner (6, 7) and, unlike the sulfonylurea class of antidiabetic drugs, replenishes insulin stores in β cells (5). In addition, GLP-1 renders β cells glucose-competent (8) and plays a role in the long-term maintenance of β cell mass. The mechanism of GLP-1’s enhancement of β cell mass seems to be multifold, with evidence that activation of the G protein-coupled GLP-1 receptor (GLP-1R) induces both neogenesis and proliferation of β cells and inhibits β cell apoptosis (9, 10). The role of GLP-1 signaling in the normal pancreas is highlighted in the GLP-1R−/− mouse, which exhibits abnormal islet architecture and mild glucose intolerance and impaired recovery from β cell insult (11). GLP-1 may thus be useful in the setting of T1D to enhance the survival and function of transplanted islet cells or to facilitate regeneration of endogenous β cells. Support for this possibility is evident in rodent models of T1D; short-term GLP-1 treatment enhanced recovery from treatment with streptozotocin (STZ), a β cell toxin, in neonatal rats (12), and immunosuppressive therapy in conjunction with transient administration of a GLP-1 mimetic induced remission in 88% of nonobese diabetic mice (13). Unfortunately, the delivery of GLP-1 by injection is hampered by the rapid degradation and clearance of the peptide (14). Moreover, cramping and nausea are common side effects of GLP-1 mimetics and limit the injected doses that are tolerable (15, 16). The doses used to promote β cell growth in rodents, typically 50–100 μg/kg of body weight (10, 13), are significantly higher than the <2 μg/kg of body weight dose that is tolerable in humans (17) and greatly exceed the dose of the GLP-1 mimetic exenatide (Byetta) that is currently prescribed to treat type 2 diabetes patients (5 μg injected twice daily). Therefore, it may not be possible to harness the β cell growth-promoting actions of GLP-1 mimetics when administered by peripheral injection.
While GLP-1 is produced from the proglucagon precursor by means of prohormone convertase (PC) 1/3 activity in enteroendocrine L cells, proglucagon is differentially processed by PC2 in α cells to release glucagon, leaving GLP-1 trapped within a larger fragment with no known function (18, 19). Thus, in the normal adult pancreas, α cells produce little GLP-1 owing to their predominant expression of the processing enzyme PC2 rather than PC1/3 (Fig. 1A). However, PC1/3 expression is activated in α cells under certain conditions, resulting in GLP-1 production. In mice, the earliest pancreatic progenitor cells express both proglucagon and PC1/3, suggesting that production of GLP-1 by these cells for a transient period might play a role in pancreatic development (20). In rodents, islet GLP-1 production is increased in response to partial pancreatectomy and induction of diabetes (21–23) and after enzymatic isolation and subsequent culture (24, 25). Thus, the liberation of GLP-1 in α cells by the expression of PC1/3 may be a natural strategy used by islets to promote islet formation and function under certain circumstances. We sought to mimic this approach by using gene transfer to induce the expression of PC1/3 in α cells (Fig. 1A) as a therapeutic strategy to improve islet function, reasoning that local production of GLP-1 directly within the islet may enable the beneficial islet actions of GLP-1 while minimizing systemic delivery and thereby unwanted side effects.
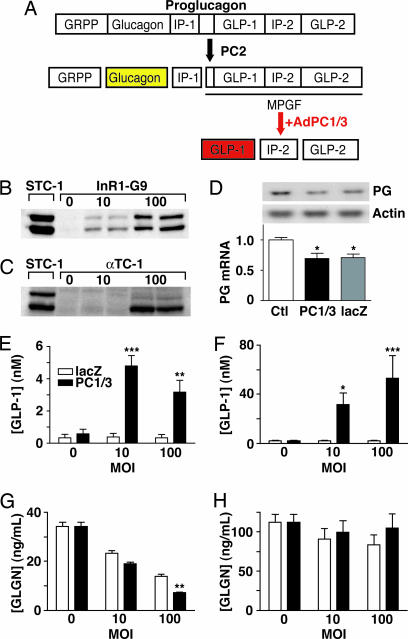
Fig. 1.
Strategy for inducing GLP-1 production in pancreatic α cells. (A) Adult pancreatic α cells liberate glucagon from the precursor proglucagon by means of PC2 activity. We hypothesized that adenoviral delivery of PC1/3 (AdPC1/3) to pancreatic α cells would liberate bioactive GLP-1 from the major proglucagon fragment (MPGF). GRPP, glicentin-related pancreatic polypeptide; IP-1 and IP-2, intervening peptides. (B and C) Western blot analysis of PC1/3 expression in control gut (STC-1) cells, InR1-G9 cells (B), and αTC-1 cells (C) 72 h after treatment with AdPC1/3 at moi = 0, 10, or 100. Blots are representative of three or more experiments. (D) Northern blot analysis of proglucagon (PG) mRNA levels in InR1-G9 cells transduced with AdPC1/3 (PC1/3) or Ad5lacZ (lacZ) (representative of three or more experiments). Data are normalized for β-actin and expressed relative to mock-transduced control (Ctl). ∗, P < 0.05 versus control using one-way ANOVA. (E–H) GLP-1 and glucagon (GLGN) secretion by InR1-G9 (E and G) and αTC-1 (F and H) cells after treatment with AdPC1/3 or Ad5lacZ at moi = 0, 10, or 100. ∗, P < 0.05; ∗ ∗, P < 0.01; ∗ ∗ ∗, P < 0.001 compared with Ad5lacZ; n ≥ 3.
Results
Transduction of Tumor-Derived α Cells with Adenovirus (Ad) PC1/3.
Using a replication-incompetent adenoviral vector carrying the CMV promoter-linked PC1/3 cDNA expression construct, we achieved a dose-dependent increase in expression of the 82-kDa form and the bioactive 64-kDa form of PC1/3 in the α cell lines InR1-G9 and αTC-1 (Fig. 1 B and C). PC1/3 overexpression induced significant accumulation of bioactive GLP-1 in the culture medium of InR1-G9 [0.58 ± 0.29 nM at multiplicity of infection (moi) = 0 versus 4.8 ± 0.64 nM at moi = 10] and αTC-1 cells (2.0 ± 0.55 nM at moi = 0 versus 53.0 ± 18.3 nM at moi = 100), whereas transduction with the reporter Ad5lacZ, bearing a β-gal construct under the control of a CMV promoter, had no impact on GLP-1 secretion (Fig. 1 E and F). Treatment with either vector at moi = 100 decreased glucagon secretion in InR1-G9 cells but not in αTC-1 cells (Fig. 1 G and H), and InR1-G9 cells transduced with either vector had decreased proglucagon mRNA levels (Fig. 1D). This decreased glucagon gene transcription and secretion is likely indicative of nonspecific viral toxicity.
Transduction of Isolated Mouse Islets with AdPC1/3.
Because α cells are concentrated in the periphery of mouse islets, we next developed conditions to achieve gene transfer to this region by using Ad5lacZ. Treatment of isolated islets with Ad5lacZ at moi = 10 resulted in a pattern of patchy, weak transduction in the α cell-rich islet periphery. Treatment at moi = 100, however, resulted in uniform transduction of virtually the entire islet periphery but not the β cells that lie in the core of the islets, except in some very small islets and islet fragments (Fig. 2A). Costaining of X-Gal-stained islets for glucagon revealed that the virus-transduced region was primarily localized to glucagon-positive areas (Fig. 2 A and B).
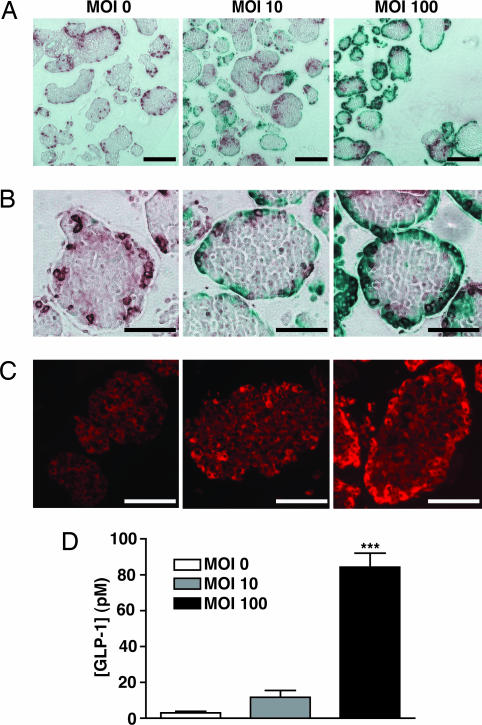
Fig. 2.
Optimization of adenoviral transduction conditions to target the mouse islet periphery and induce islet GLP-1 secretion. (A and B) Ad5lacZ was used to optimize transduction conditions such that the islet periphery was transduced in isolated islets. Transduced cells appear blue; glucagon-positive cells appear brown. (C) PC1/3 immunoreactivity in mouse islets 72 h after treatment with AdPC1/3. (Scale bars: A, 250 μm; B and C, 100 μm.) (D) GLP-1 secretion by mouse islets 72 h after treatment with AdPC1/3. Data were analyzed by using one-way ANOVA; ∗ ∗ ∗, P < 0.001 compared with moi = 0; n = 3.
Immunostaining showed that by using the same gene transfer conditions with AdPC1/3, we were successful in introducing PC1/3 to cells in the islet periphery, increasing the levels of PC1/3 immunoreactivity in the periphery to exceed those in the islet β cell core, which expresses this enzyme endogenously (Fig. 2C). Consistent with our results in InR1-G9 and αTC-1 cells (Fig. 1 E and F), increased peripheral PC1/3 expression in islets significantly increased bioactive GLP-1 release, by a magnitude of up to 29 times (Fig. 2D; 84.3 ± 7.9 pM at moi = 100 versus 2.9 ± 0.9 pM for control islets).
We next examined the impact of increased islet GLP-1 production on islet function and survival. Mock- or AdPC1/3-transduced islets were challenged with high (20 mM) glucose, and glucose-stimulated insulin output was assessed in a perifusion system. AdPC1/3-transduced islets (moi = 100) had a greater insulin response to glucose stimulation than mock-transduced islets, with greater peak insulin levels (Fig. 3A; 4.76 ± 1.15 ng/ml versus 2.63 ± 0.55 ng/ml) and a 57% increase in total insulin output (Fig. 3A Inset; 88.2 ± 20.69 versus 56.27 ± 17.0 ng/ml per min).
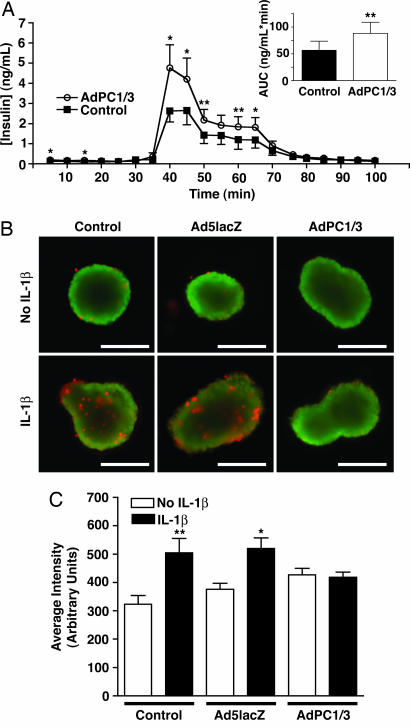
Fig. 3.
AdPC1/3 transduction enhances glucose-stimulated insulin secretion and survival of isolated mouse islets. (A) Glucose-stimulated insulin secretion in mouse islets 72 h after transduction with AdPC1/3 (moi = 100). Area under curve (AUC; Inset) was determined for the entire curve normalized for baseline. Data were analyzed with a one-tailed t test; n = 4 with two independent replicates per experiment. (B and C) AdPC1/3-transduced islets are protected against 24-h IL-1β treatment. (B) Merged image of live (green) and dead (red) cells in whole mouse islets treated with AdPC1/3 or Ad5lacZ (moi = 10) and with or without IL-1β (50 units/ml). (Scale bar: 200 μm.) (C) Quantification of cell death in islets treated with IL-1β, reported as average red fluorescence intensity normalized for the islet area. Data were collected from 6–15 individual islets per condition for two independent experiments and analyzed by using one-way ANOVA. ∗, P < 0.05; ∗ ∗, P < 0.01.
We also examined whether transduction with AdPC1/3 offered islets protection against IL-1β, which induces β cell apoptosis and may be involved in islet cell death in the early stages after islet transplantation (26). Although there was no detectable difference in the number of live cells in islets that received virus and/or cytokine treatment compared with untreated islets, there was a trend toward increased cell death in virus-transduced islets (both AdPC1/3 and Ad5lacZ); however, this trend did not reach statistical significance (Fig. 3 B and C). This cell death is probably due to nonspecific toxicity associated with adenoviral transduction. Treatment with IL-1β induced a significant increase in cell death in mock-transduced and Ad5lacZ-transduced islets. Remarkably, however, AdPC1/3-transduced islets showed no increase in cell death upon exposure to IL-1β and thus appeared to be protected against IL-1β-induced cell death (Fig. 3 B and C).
Transplantation of AdPC1/3-Transduced Mouse Islets.
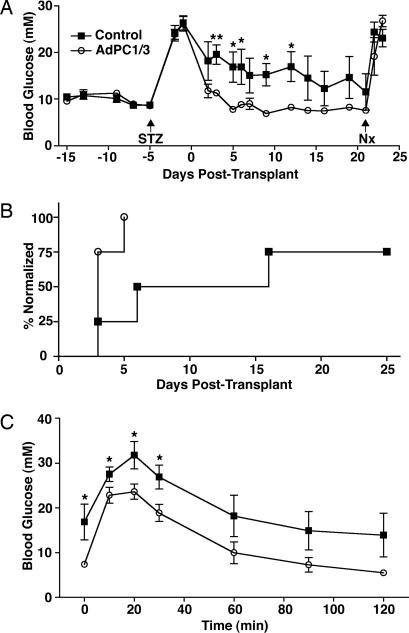
Having established that AdPC1/3-transduced islets have enhanced function and survival in vitro, we next tested the performance of these GLP-1-producing islets in a marginal mass model of islet transplantation in mice. Because transduction of islets with a high dose of virus in vitro was associated with a slight increase in cell death (Fig. 3C), we opted to test a lower dose of virus (moi = 10) in vivo. After induction of diabetes with STZ, mice received a subcapsular transplant of 200 nontransduced control islets or AdPC1/3-transduced islets. Control islets were slow to restore normoglycemia in recipient mice or were completely incapable of ameliorating hyperglycemia. In marked contrast, however, transplantation of AdPC1/3-transduced islets resulted in a prompt return to normoglycemia in all recipients (Fig. 4A and B). When recipient mice were challenged with an i.p. glucose tolerance test 5 d after transplant, mice that had received AdPC1/3-treated islets demonstrated better glucose tolerance at all time points (Fig. 4C). There was no effect of AdPC1/3 treatment of islets on the body weight of recipient animals (data not shown). There also did not seem to be any deleterious, nonspecific effects of virus transduction on the long-term function of islet grafts (or any such effects were masked by the benefits of GLP-1 production), because all recipient mice maintained normoglycemia for the duration of the study. All animals reverted to diabetes upon removal of the islet graft-bearing kidney 2 d (data not shown) or 21 d (Fig. 4A) after transplant.
Fig. 4.
AdPC1/3 transduction improves islet transplantation outcomes in STZ-treated mice. STZ-treated diabetic mice received subcapsular transplant of mock- (control) or AdPC1/3-transduced (moi = 10) islets on day 0 and nephrectomy (Nx) on day 21. (A) Blood glucose levels in transplant recipients (n = 4). (B) Blood glucose normalization curves for transplant recipients, expressed as percentage of animals normalized. The day of normalization was considered the second of ≥2 consecutive days where blood glucose was <15 mM. (C) i.p. glucose tolerance test (2 g/kg) performed 5 d after transplantation. Data were analyzed by using a two-tailed t test. ∗, P < 0.05; ∗ ∗, P < 0.01.
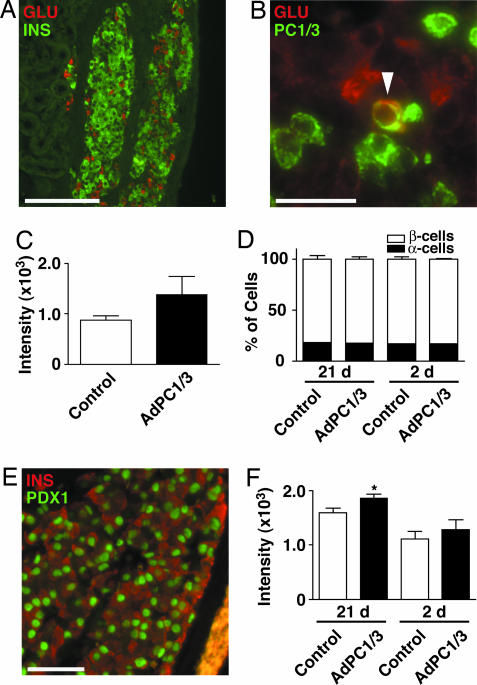
Histological examination was performed on grafts retrieved from mice. Both α and β cells were easily identified by robust glucagon and insulin immunofluorescence (Fig. 5A), although PC1/3 expression was not detectable in α cells of grafts harvested 21 d after transplant (data not shown). There was a trend toward more intense insulin staining in β cells of AdPC1/3-transduced grafts, but this trend did not reach statistical significance (Fig. 5C). Islet grafts harvested 2 d after transplantation exhibited weaker insulin staining and β cell degranulation (data not shown). However, a subset of α cells was clearly PC1/3- and glucagon-positive at this time point (arrowhead, Fig. 5B). There was no difference in the proportion of α cells or β cells in control versus AdPC1/3-transduced islet grafts at either 2 or 21 d after transplant (Fig. 5D). Because GLP-1’s effects on β cells include enhanced expression and activity of the transcription factor Pdx1 (27), we performed double immunofluorescence for Pdx1 and insulin in islet grafts. We could not detect significant cytoplasmic Pdx1 staining in either control or AdPC1/3-transduced grafts (Fig. 5E). However, nuclear Pdx1 staining was more intense in β cells in AdPC1/3-transduced grafts compared with controls (Fig. 5F), although this effect was significant only for the grafts harvested 21 d after transplant.
Fig. 5.
Analysis of AdPC1/3-transduced islet grafts after nephrectomy. Islet grafts were removed 2 or 21 d after transplantation and sectioned for analysis. (A) Representative graft costained for glucagon (red) and insulin (green). (B) Glucagon (red) and PC1/3 (green) staining in a representative graft retrieved 2 d after transplantation. (C) Quantification of the intensity of insulin staining in islet grafts (21 d after transplantation). Data are presented as mean fluorescence intensity; n ≥ 3. (D) Proportion of α and β cells in islet grafts costained for insulin and glucagon, expressed as the percentage of total cells counted; n ≥ 3. (E) Representative islet graft costained for insulin (red) and Pdx1 (green). (Scale bars: A, 190 μm; B and E, 50 μm.) (F) Quantification of the intensity of nuclear Pdx1 staining in islet grafts. Data are reported as mean fluorescence intensity and were analyzed by a one-tailed t test with n = 3. ∗, P < 0.05.
Discussion
Here, we have shown that up-regulation of PC1/3 expression in α cells enhances the survival and function of islets. Prevailing dogma has been that PC1/3 is not produced in adult islet α cells, precluding GLP-1 production. However, we could detect bioactive GLP-1 production in InR1-G9 and αTC-1 cells, and GLP-1 also was secreted at low levels in normal, nontransduced islets, consistent with several reports that showed that islet α cells do produce small amounts of fully processed GLP-1, which may act as a paracrine stimulus on β cells (24, 25). As with the α cell lines, we were able to significantly increase GLP-1 output from native islet α cells by increasing PC1/3 expression. Although the PC1/3 gene was likely transferred to a small number of peripheral β cells in addition to α cells, we did not anticipate adverse effects of such inadvertent delivery, because β cells endogenously express PC1/3. Indeed, AdPC1/3-transduced islets had enhanced glucose-induced insulin secretion and were protected from IL-1β-mediated cell death, consistent with known actions of GLP-1. Furthermore, after transplantation of an equivalent mass of islets, the ability of AdPC1/3-treated islets to restore good glucose control was superior to that of normal islets, with fasting blood glucose and glucose tolerance that matched that observed in normal mice.
How might delivery of PC1/3 to islet α cells lead to greater transplantation success in diabetic mice? We propose that α cell-derived GLP-1 may interact with its receptor on the β cell surface by diffusion through the interstitial space. The microcirculation of the native and transplanted islet remains controversial (28); it is also possible that GLP-1 reaches β cells by means of direct vascular connections. The insulinotropic effect of GLP-1 could help restore normoglycemia sooner in transplant recipients and thereby relieve the glucotoxicity associated with prolonged exposure of islet grafts to high glucose levels (4). However, because secretion from α cells is increased at low glucose levels, the improved transplantation outcomes that are observed may be primarily due to promotion of β cell survival and function by means of continuous exposure of islets to local GLP-1 during postabsorptive conditions. GLP-1 is known to activate insulin gene transcription (5), and although we did not directly measure insulin content, there was a trend toward increased insulin immunofluorescence in AdPC1/3-transduced islet grafts. In addition, it has recently been reported that GLP-1’s effects on β cell growth and survival are mediated by means of phosphorylation-dependent nuclear exclusion of FoxO1, which in turn allows increased expression of the transcription factors Pdx1 and Foxa2 (29). Indeed, we did observe greater nuclear Pdx1 staining in the β cells of AdPC1/3-transduced grafts, suggesting that Pdx1 expression is up-regulated in these islets and may contribute to the marked improvement of transplant outcomes.
It is probable that the introduction of PC1/3 to islet α cells allowed not only GLP-1 release but also GLP-2 release from the major proglucagon fragment, because PC1/3 is responsible for the release of both of these peptides in gut L cells (19). GLP-2’s primary actions are thought to be exerted in the gut as a growth factor for crypt cells and enterocytes (5). Because the GLP-2 receptor has not been localized to the pancreatic islet (30), and, further, because GLP-2 is not known to have an insulinotropic effect (31), we consider it highly unlikely that the effects observed in the present study could be attributed to GLP-2 rather than GLP-1 production. Nevertheless, studies with GLP-1R antagonists or GLP-1R knockout mice would be required to definitively establish the contribution of GLP-1R signaling to the observed improvements in islet function and survival.
Islet transplantation markedly improves the lives of patients with diabetes, but, unfortunately, this approach typically requires islets from multiple donors and supplementation with exogenous insulin (2). Preventing failure of transplanted islets is thus important not only to improve transplantation outcomes but also to reduce the number of islets required per recipient and thereby ease the burden on the limited supply of available tissue. In humans, α cells are found scattered throughout the islet, whereas in rodents, they are generally limited to the islet periphery. However, the greater proportion of α cells in human islets compared with mouse islets (32) means that the absolute number of α cells available for transduction in the islet periphery may be similar in rodents and humans. With the aid of α cell-specific gene delivery vectors and/or the optimization of transduction conditions for human islets, inducing local GLP-1 production within islets as described here may offer a unique approach for improving the outcome of islet transplantation in humans. It remains an intriguing possibility that this strategy could also be exploited to intervene in the loss of a functional mass of β cells, which characterizes diabetes. This hypothesis can now be tested, because techniques have been developed to deliver genes to islets in vivo (33).
Materials and Methods
Islet isolation and tissue culture media, antibiotics, FBS, X-Gal, the live/dead assay kit (L-3224), TRIzol, and secondary antibodies used for immunohistochemistry were obtained from Invitrogen (Burlington, Canada). Collagenase, BSA, glutaraldehyde, protease inhibitor mixture, and goat anti-rabbit Cy3 and mouse anti-glucagon antisera were from Sigma-Aldrich (Oakville, Canada). Assay kits (Active GLP-1 ELISA, Sensitive Insulin RIA, and Glucagon RIA), guinea pig anti-insulin antibody, and dipeptidyl peptidase IV inhibitor were from Linco Research (St. Charles, MO). Reagents for Western blotting were obtained from GE Healthcare (formerly Amersham Pharmacia Biosciences, Buckinghamshire, U.K.). αTC-1 (clone 9) cells were obtained from the American Type Culture Collection (Manassas, VA). Imaging and quantification for immunocytochemistry and for the live/dead assay were performed by using an Axiovert 200 microscope (Zeiss, Toronto, Canada) connected to a digital camera (Retiga 2000R, QImaging, Burnaby, Canada) controlled with openlab 3.0 software (Improvision, Lexington, MA). AdPC1/3 and Ad5lacZ are described in refs. 34 and 35.
Adenoviral Transduction and Analysis of Cultured Cells.
InR1-G9, αTC-1, and STC-1 (enteroendocrine) cells were cultured in high-glucose DMEM containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Dipeptidyl peptidase IV inhibitor (10 μl/ml) was included in all media. Cells were transduced with AdPC1/3 or Ad5lacZ at moi = 10 or 100 at 37°C and 5% CO2 for 2 h. A complete medium change was performed every 24 h for 3 d, and the 48- to 72-h medium samples were assayed for glucagon or GLP-1. Three days after transduction, RNA was isolated by using the TRIzol method, or cell lysates were prepared. Equal amounts of protein were electrophoresed and transferred to a 0.2-μm poly(vinylidene difluoride) membrane (Bio-Rad, Hercules, CA). After blocking, the membrane was incubated with rabbit anti-PC1/3 antiserum (1:1,000) and developed by using horseradish peroxidase-conjugated secondary antibody (1:5,000). For Northern blotting, RNA was electrophoresed on a 1.5% denaturing agarose gel, transferred to a 0.45-μm nitrocellulose membrane, and UV-crosslinked. [32P]-labeled β-actin (Ambion, Austin, TX) and rat proglucagon cDNA probes were prepared by the random-priming method. Densitometric analysis was performed by using imagequant 5.2 (Amersham Pharmacia Biosciences, Piscataway, NJ).
Mouse Islet Transduction and Analysis.
CD1 and C57BL/6 mice were obtained from the University of British Columbia (UBC) Animal Care Facility and The Jackson Laboratory (Bar Harbor, ME), respectively, and all experiments were approved by the UBC Animal Care Committee. Islets were isolated from male mice (age 8–12 weeks) by injection of collagenase (1,000 units/ml in Hanks’ balanced salt solution). Pancreata were gently shaken at 37°C for 10–13 min, and islets were purified according to standard procedure. Islets were cultured in Ham’s F-10 containing 0.5% BSA, 6.1 mM glucose, 100 units/ml penicillin, and 100 μg/ml streptomycin.
One day after isolation, islets (300 per condition) were mock-transduced or transduced with Ad5lacZ or AdPC1/3 at moi = 10 or 100 (assuming 1,000 cells per islet) for 1 h at 37°C and 5% CO2. Three days after transduction, Ad5lacZ-transduced islets were fixed in 0.2% glutaraldehyde, exposed to X-Gal for 2 h at 37°C, and paraffin-embedded. Sections were incubated with mouse anti-glucagon (1:1,800) and anti-mouse horseradish peroxidase-conjugated (1:800) antibodies and then developed by using a diaminobenzidine substrate kit. Other islets were transduced as above and cultured for 3 d with a daily complete medium change, and 48- to 72-h medium samples were assayed for GLP-1 and glucagon. Three days after transduction, islets were fixed in 4% paraformaldehyde and embedded in paraffin, and sections were immunostained with rabbit anti-PC1/3 antiserum (1:900, Affinity BioReagents, Golden, CO) and goat anti-rabbit-Cy3 antiserum (1:800).
Perifusion of Mouse Islets.
Islets were loaded into temperature-/CO2-controlled chambers (100 islets per chamber) of an Acu-syst S Perifusion apparatus (Endotronics, Coon Rapids, MN) 3 d after transduction with AdPC1/3 (moi = 100). Hepes-buffered Krebs–Ringer bicarbonate buffer containing 0.5% BSA and 3 mM or 20 mM glucose was pumped through the chambers at ≈2 ml/min after a 1-h preincubation under basal conditions. Fractions were collected every 5 min and assayed for insulin.
Cytokine Treatment and Live/Dead Imaging of Mouse Islets.
Forty-eight hours after transduction, 12–25 AdPC1/3- or Ad5lacZ-transduced islets (moi = 10) were handpicked and placed in wells of a black 96-well plate. Some wells contained 50 units/ml IL-1β (R & D Systems, Minneapolis, MN), and islets were cultured for an additional 24 h. Analysis of live and dead cells was performed according to the manufacturer’s instructions by using a kit in which ethidium homodimer 1 fluoresces red in cells with damaged membranes and calcein-AM is converted to a product fluorescing in the green range in living cells. Images were captured at the central plane of focus on both red and green channels and merged. Mean red fluorescence intensity was determined by normalizing total internal islet red fluorescence for the islet area.
Transplantation of Mouse Islets.
Tail vein blood glucose was measured by using a handheld glucometer (Lifescan, Mountain View, CA) after a 4-h morning fast. STZ (200 mg/kg; prepared in citrate buffer with pH = 4.5) was delivered by single i.p. injection 5 d before transplantation, and diabetes was confirmed by blood glucose readings >20 mM on at least 2 consecutive days. Isolated islets were mock-transduced or transduced with AdPC1/3 at moi = 10. The following day, 200 islet aliquots were handpicked and transplanted under the capsule of the left kidney of anaesthetized recipient mice. An i.p. glucose tolerance test (2 mg/kg) was performed after a 4-h morning fast.
Two or 21 d after transplantation, survival nephrectomy was performed to remove the islet graft-bearing kidney, and blood glucose was monitored for several days to confirm recurrence of hyperglycemia. Islet grafts were fixed in 4% paraformaldehyde and paraffin-sectioned. Sections were incubated with rabbit anti-Pdx1 (1:1,000), guinea pig anti-insulin (1:900), mouse anti-glucagon (1:1,800), and/or rabbit anti-PC1/3 (1:1,000) antisera and secondary antisera conjugated to Alexa Fluor 488 or 594 (1:800). Some sections were costained for insulin and Pdx1, and the intensity of nuclear Pdx1 staining was quantified in 10 randomly selected insulin-positive cells from each of four nonoverlapping fields of view for each graft. Intensity of insulin staining and the relative proportions of α/β cells were determined in sections costained for insulin and glucagon. Intensity of insulin staining was determined in seven or more nonoverlapping fields of view for each graft. The proportion of α and β cells was determined by counting nuclei of all glucagon- and insulin-positive cells in three to eight nonoverlapping fields of view for each graft.
Statistical Analysis.
Data are presented as mean ± SEM and were analyzed by using Student’s t test or ANOVA with a Bonferroni post test as appropriate.
Acknowledgments
We thank Dr. J.-C. Irminger and Ms. K. Rickenbach (University of Geneva, Geneva, Switzerland) for providing AdPC1/3, Dr. P. Brubaker (University of Toronto, Toronto, Canada) for InR1-G9 cells, Dr. D. Drucker (University of Toronto) for STC-1 cells and the proglucagon probe, Dr. L. Devi (Mount Sinai School of Medicine, New York, NY) and Dr. J. Habener (Harvard University, Boston, MA) for providing anti-PC1/3 and anti-Pdx1 antisera, respectively, and Ms. M. Speck for excellent technical support. This work was supported by a grant from the Juvenile Diabetes Research Foundation, Career Development Awards from the Juvenile Diabetes Research Foundation (to T.J.K. and J.D.J.), scholarships from the Michael Smith Foundation for Health Research (to T.J.K., J.D.J., and R.D.W.), and scholarships from the Natural Sciences and Engineering Research Council (to R.D.W.).
Abbreviations
- GLP-1
glucagon-like peptide 1
- PC
prohormone convertase
- T1D
type 1 diabetes
- moi
multiplicity of infection
- STZ
streptozotocin
- Ad
adenovirus
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Shapiro A. M., Lakey J. R., Ryan E. A., Korbutt G. S., Toth E., Warnock G. L., Kneteman N. M., Rajotte R. V. N. Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Robertson R. P. N. Engl. J. Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 3.Davalli A. M., Scaglia L., Zangen D. H., Hollister J., Bonner-Weir S., Weir G. C. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 4.Biarnes M., Montolio M., Nacher V., Raurell M., Soler J., Montanya E. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 5.Kieffer T. J., Habener J. F. Endocr. Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 6.Mojsov S., Weir G. C., Habener J. F. J. Clin. Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreymann B., Williams G., Ghatei M. A., Bloom S. R. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 8.Holz G. G. T., Kuhtreiber W. M., Habener J. F. Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu G., Stoffers D. A., Habener J. F., Bonner-Weir S. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Hansotia T., Yusta B., Ris F., Halban P. A., Drucker D. J. J. Biol. Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 11.Ling Z., Wu D., Zambre Y., Flamez D., Drucker D. J., Pipeleers D. G., Schuit F. C. Virchows Arch. 2001;438:382–387. doi: 10.1007/s004280000374. [DOI] [PubMed] [Google Scholar]
- 12.Tourrel C., Bailbe D., Meile M. J., Kergoat M., Portha B. Diabetes. 2001;50:1562–1570. doi: 10.2337/diabetes.50.7.1562. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa N., List J. F., Habener J. F., Maki T. Diabetes. 2004;53:1700–1705. doi: 10.2337/diabetes.53.7.1700. [DOI] [PubMed] [Google Scholar]
- 14.Kieffer T. J., McIntosh C. H., Pederson R. A. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 15.Elbrond B., Jakobsen G., Larsen S., Agerso H., Jensen L. B., Rolan P., Sturis J., Hatorp V., Zdravkovic M. Diabetes Care. 2002;25:1398–1404. doi: 10.2337/diacare.25.8.1398. [DOI] [PubMed] [Google Scholar]
- 16.Kolterman O. G., Buse J. B., Fineman M. S., Gaines E., Heintz S., Bicsak T. A., Taylor K., Kim D., Aisporna M., Wang Y., Baron A. D. J. Clin. Endocrinol. Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 17.Calara F., Taylor K., Han J., Zabala E., Carr E. M., Wintle M., Fineman M. Clin. Ther. 2005;27:210–215. doi: 10.1016/j.clinthera.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Rouille Y., Martin S., Steiner D. F. J. Biol. Chem. 1995;270:26488–26496. doi: 10.1074/jbc.270.44.26488. [DOI] [PubMed] [Google Scholar]
- 19.Dhanvantari S., Seidah N. G., Brubaker P. L. Mol. Endocrinol. 1996;10:342–355. doi: 10.1210/mend.10.4.8721980. [DOI] [PubMed] [Google Scholar]
- 20.Wilson M. E., Kalamaras J. A., German M. S. Mech. Dev. 2002;115:171–176. doi: 10.1016/s0925-4773(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 21.Thyssen S., Arany E., Hill D. J. Endocrinology. 2006;147:2346–2356. doi: 10.1210/en.2005-0396. [DOI] [PubMed] [Google Scholar]
- 22.De Leon D. D., Deng S., Madani R., Ahima R. S., Drucker D. J., Stoffers D. A. Diabetes. 2003;52:365–371. doi: 10.2337/diabetes.52.2.365. [DOI] [PubMed] [Google Scholar]
- 23.Nie Y., Nakashima M., Brubaker P. L., Li Q. L., Perfetti R., Jansen E., Zambre Y., Pipeleers D., Friedman T. C. J. Clin. Invest. 2000;105:955–965. doi: 10.1172/JCI7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller R. S., Aponte G. W. Am. J. Physiol. 1995;269:G852–G860. doi: 10.1152/ajpgi.1995.269.6.G852. [DOI] [PubMed] [Google Scholar]
- 25.Masur K., Tibaduiza E. C., Chen C., Ligon B., Beinborn M. Mol. Endocrinol. 2005;19:1373–1382. doi: 10.1210/me.2004-0350. [DOI] [PubMed] [Google Scholar]
- 26.Corbett J. A., Wang J. L., Sweetland M. A., Lancaster J. R., Jr., McDaniel M. L. J. Clin. Invest. 1992;90:2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buteau J., Roduit R., Susini S., Prentki M. Diabetologia. 1999;42:856–864. doi: 10.1007/s001250051238. [DOI] [PubMed] [Google Scholar]
- 28.Brunicardi F. C., Stagner J., Bonner-Weir S., Wayland H., Kleinman R., Livingston E., Guth P., Menger M., McCuskey R., Intaglietta M., et al. Diabetes. 1996;45:385–392. doi: 10.2337/diab.45.4.385. [DOI] [PubMed] [Google Scholar]
- 29.Buteau J., Spatz M. L., Accili D. Diabetes. 2006;55:1190–1196. doi: 10.2337/db05-0825. [DOI] [PubMed] [Google Scholar]
- 30.Yusta B., Huang L., Munroe D., Wolff G., Fantaske R., Sharma S., Demchyshyn L., Asa S. L., Drucker D. J. Gastroenterology. 2000;119:744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt W. E., Siegel E. G., Creutzfeldt W. Diabetologia. 1985;28:704–707. doi: 10.1007/BF00291980. [DOI] [PubMed] [Google Scholar]
- 32.Cabrera O., Berman D. M., Kenyon N. S., Ricordi C., Berggren P. O., Caicedo A. Proc. Natl. Acad. Sci. USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z., Zhu T., Rehman K. K., Bertera S., Zhang J., Chen C., Papworth G., Watkins S., Trucco M., Robbins P. D., et al. Diabetes. 2006;55:875–884. doi: 10.2337/diabetes.55.04.06.db05-0927. [DOI] [PubMed] [Google Scholar]
- 34.Irminger J. C., Meyer K., Halban P. Biochem. J. 1996;320:11–15. doi: 10.1042/bj3200011. Part 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Cheung A. T., Kolls J. K., Starks W. W., Martinez-Hernandez A., Dietzen D., Bryer-Ash M. Diabetes Obes. Metab. 2001;3:367–380. doi: 10.1046/j.1463-1326.2001.00173.x. [DOI] [PubMed] [Google Scholar]