Abstract
Human B lymphocytes and derived lines from a spectrum of B cell malignancy were studied for expression of dopaminergic pathway components and for their cytostatic response to the catecholamine and related, potentially therapeutic compounds. Proliferating normal lymphocytes and dividing malignant clones rapidly arrested on exposure to dopamine in the low (≤10 μM) micromolar range. The antiparkinsonian drugs l-DOPA and apomorphine (particularly) were similarly antiproliferative. With the exception of D4, dopamine receptors D1–D5 were variably expressed among normal and neoplastic B cell populations, as was the dopamine transporter. Transcripts for D1 and D2 were frequently found, whereas D3 and D5 revealed restricted expression; dopamine transporter was detected in most cases. Nevertheless, pharmacological analysis disclosed that dopamine targeted cycling B cells independent of these structures. Rather, oxidative stress constituted the primary mechanism: the catecholamine’s actions being mimicked by hydrogen peroxide and reversed by exogenous catalase, and evidence for the intracellular redox protein thioredoxin contributing protection. Among proliferating clones, growth arrest was accompanied by cell death in populations deplete in antiapoptotic Bcl-2: resting lymphocytes escaping low micromolar dopamine toxicity. Dysregulated bcl-2 expression, although preventing oxidative-induced caspase-dependent apoptosis, by itself conferred only minor protection against dopamine cytostasis. The selective impact of dopamine on lymphocytes that are in active cycle indicates an axis for therapeutic intervention not only in B cell neoplasia but also in lymphoproliferative disturbances generally. Rational tailoring of drug delivery systems already in development for Parkinson’s disease could provide ideal vehicles for carrying the oxidative hit directly to the target populations.
Keywords: apoptosis, oxidative stress, Parkinson’s disease, Bcl-2, monoamines
The non-Hodgkin’s lymphomas (NHL) comprise a heterogeneous group of malignancy, the overwhelming majority of which arise from oncogenic transformation of B cells (1). Although it is a disease increasing in frequency, continuing advances are being made in its treatment, currently exemplified by humanized anti-CD20 (Rituximab) (2). There remain, however, significant subsets of NHL where clinical responses are limited. At the extreme is CNS lymphoma, where mean survival even with radical therapy stands at <2 years; among individuals with AIDS the outlook is even worse (3). The HIV-positive population has also seen NHL become the major AIDS-defining illness for which survival remains severely compromised in the highly active antiretroviral therapy era (4). Almost half the NHL in this cohort is now of the highly aggressive Burkitt’s type with median overall survival of ≈8 months compared with 22 months for diffuse large B cell lymphoma (5). Burkitt’s lymphoma (BL) also demands more widely available and cheaper treatment in the world’s malarial belts, where it was first defined and remains endemic (6, 7).
In response to the need for novel modalities in treating NHL, we have turned our attention to the neuroimmune axis as a potential source of fresh targets. Not only is there increasing acceptance of a constant communication between immune and nervous systems, but also (and of particular relevance here) the molecular elements providing the syntax to the language used are being deciphered (8–10). The identifying of classically considered neurotrophic constituents within the immune system opens up the component cells, and their pathologies, to modulation by drugs developed principally for CNS disturbances. An example of this is the finding that BL cells carry the serotonin transporter, which in turn acts as a conduit for serotonin-driven apoptosis, leading to the discovery that widely used antidepressants deliver analogous outcomes in a spectrum of NHL (11–13).
The present study scrutinizes the dopaminergic pathway as an additional resource to supplement the NHL therapeutic arsenal. A growing literature highlights the potential for immune cells to express both the dopamine (DA) transporter (DAT) and at least a complement of the five identified DA receptors (14, 15). Meanwhile, there is continuing development of drugs and delivery systems to target dopaminergic components at pathological sites spurred by the progressive, and currently irreversible, debilitation that accompanies Parkinson’s disease (PD) (16). With these considerations in mind, we define here for both normal B cells and their lymphoma counterparts (i) the expression profile of potential dopaminergic targets; (ii) the impact of DA and related antiparkinsonian drugs on population dynamics; (iii) the pathway of DA’s action; and (iv) potential resistance mechanisms to the desired outcome. The findings indicate DA for consideration in the therapeutic targeting of NHL.
Results
DA Is Antiproliferative for Normal and Malignant B Cells at Low Micromolar Concentrations.
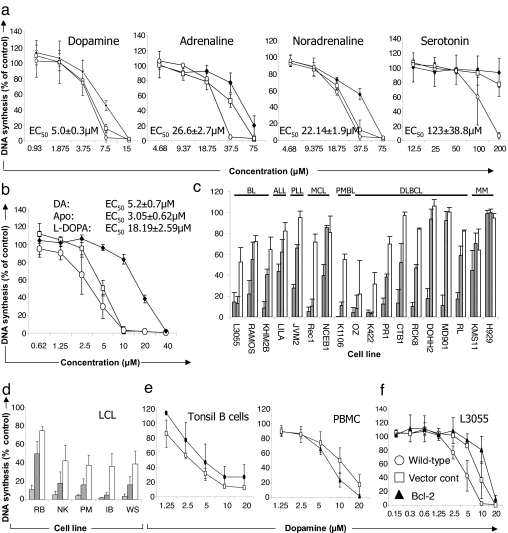
DA, adrenaline, noradrenaline, and serotonin are monoamines involved in regulating both neuronal and nonneuronal cells, including those of the immune system. Fig. 1a illustrates their effect on the proliferation (DNA synthesis at 24 h) of the L3055 BL line plated at three cell densities. DA is the most potent, with an EC50 value of 5 ± 0.3 μM for growth cessation at the lowest cell density (2.5 × 105 per milliliter), followed by (in order of potency) noradrenaline = adrenaline > serotonin. The antiparkinsonian drugs 3,4-dihydroxy-l-phenylalanine (l-DOPA) and apomorphine were similarly antiproliferative (Fig. 1b), apomorphine being slightly more potent than DA itself. Moreover, with the one exception of the multiple myeloma line H929, 5–20 μM DA delivered significant cytostasis to a spectrum of B cell malignancies representing stages of maturation arrest from pre-B cells through to plasma cells (Fig. 1c). Proliferating nonmalignant B cells were also sensitive: lymphoblastoid cell lines (LCL) (Fig. 1d) and mitogen-stimulated tonsil B cells (Fig. 1e) each demonstrated markedly suppressed proliferative responses to low micromolar DA as did polyclonally stimulated peripheral blood mononuclear cells (PBMC).
Fig. 1.
Antiproliferative effects of monoamines and related compounds against human malignant B cell lines and proliferating normal lymphocytes. Cells at 5 × 104 per milliliter (unless indicated) were incubated (for 24 h unless indicated), and proliferation was assessed by [3H]thymidine incorporation over the last 4 h. Data are presented as cpm (percentage of control) with mean ± SD from three separate experiments. (a) Effect of monoamines against L3055 BL cells at 105 per milliliter (♦), 5 × 104 per milliliter (□), or 2.5 × 104 per milliliter (○). EC50 values are indicated for cells at 2.5 × 104 per milliliter. (b) Effects of DA (□) l-DOPA (♦), and apomorphine (○) against L3055 cells with EC50 values. (c) Effect of 5 μM (open bars), 10 μM (hatched bars), or 20 μM (shaded bars) DA on proliferation of malignant B cell lines. (d) As for c, but against LCL cells at 2.5 × 104 per milliliter. (e) Effect of DA on normal proliferating lymphocytes (measured on day 3). PBMC were stimulated with phytohemagglutinin (10 μg/ml; ▴) or phorbol 12-myristate 13-acetate (2 ng/ml) plus ionomycin (1 μg/ml; □). Tonsillar B cells were stimulated with phorbol 12-myristate 13-acetate plus ionomycin (□) or soluble CD40L (1 μg/ml; ▴). (f) Effect of overexpressing Bcl-2 in L3055 cells. Apo, apomorphine; ALL, acute lymphocytic leukemia; PLL, prolymphocytic leukemia; MCL, mantle cell lymphoma; PMBL, primary mediastinal B cell lymphoma; DLBCL, diffuse large B cell lymphoma; MM, multiple myeloma.
It can be seen from Fig. 1c that L3055 is one of the more DA-sensitive lines. BL cells are characteristically low in antiapoptotic Bcl-2 (17), so we asked whether overexpressing the survival protein conferred resistance to DA’s cytostatic potential. This possibility was examined by comparing wild-type L3055 cells (WTL3055) with those stably expressing bcl-2 (L3/Bcl-2) or the empty transfection vector (L3/VC). Enforced expression of Bcl-2 offered, at best, limited protection from DA’s antiproliferative actions (Fig. 1f).
DA Can Promote Apoptosis in Cycling B Cells While Sparing Resting Lymphocytes.
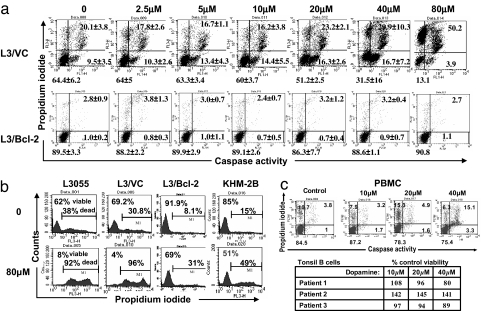
The L3055 “set” was next exposed to DA for 6 h and then dual-stained with propidium iodide (PI) and phiphilux, a caspase substrate serving as a marker of apoptosis (12). Fig. 2a reveals that, in L3/VC (and in WTL3055; data not shown), 6 h of treatment with increasing DA concentrations leads to steadily enhanced apoptosis as demonstrated by a decrease in the percentage of viable cells (caspaselo/PIlo) with a concomitant increase in early apoptotic (caspasehi/PIlo), late apoptotic (caspasehi/PIhi), and, eventually, necrotic (caspaselo/PIhi) cells. Conversely, in L3/Bcl-2 cells no caspase activation was observed even with DA at 80 μM. Prolonged exposure to high catecholamine concentration does, however, begin to prompt necrosis in Bcl-2-overexpressing cells as registered by PI uptake (Fig. 2b). This change is observed for both the L3/Bcl-2 transfectant and KHM-2B, an unusual BL line carrying (in addition to the hallmark 8:14 myc-translocation) a 14:18 translocation juxtaposing bcl-2 to the IgH chain locus (18).
Fig. 2.
Apoptosis and viability of L3055 cells in response to DA. (a) L3055 cells containing empty transfection vector (L3/VC) or containing the bcl-2 gene (L3/Bcl-2) were seeded at 2.5 × 105 per milliliter and treated with or without DA at the concentrations indicated for 6 h. Cells were dual-stained with PI and caspase substrate phiphilux and analyzed by flow cytometry. Dot plots are representative of three experiments showing the percentage of viable (caspaselo/PIlo), early apoptotic (caspasehi/PIlo), late apoptotic (caspasehi/PIhi), and necrotic (caspaselo/PIhi) cells. (b) Viability of (wild-type) WTL3055, L3/VC, L3/Bcl-2, or KHM-2B (2.5 × 105 per milliliter) cells in response to prolonged (24-h) DA exposure as assessed by PI staining. (c) Viability of resting PBMC or tonsil B cells (5 × 105 per milliliter) in response to DA for 24 h assessed as in a. (Upper) PBMC. (Lower) Percentage (of control) viable cells (caspaselo/PIlo) for B cells from three individual tonsils.
Resting cell viability (PBMC or tonsil B cells) was relatively uncompromised even after 24 h of exposure to 40 μM DA (Fig. 2c), a concentration that prompts them to full growth arrest when cycling (Fig. 1e). Such resistance contrasts markedly with L3055 wild-type cells, registering as early as 6 h on encountering 40 μM DA a 55.3 ± 25% decrease in viability compared with control values.
Normal and Malignant B Cells Express Dopaminergic Components, but Growth Arrest Is Independent of These Targets.
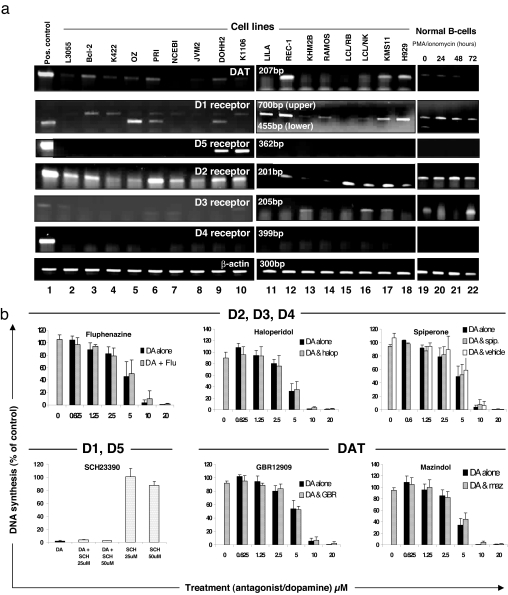
Selecting malignant lines, LCL, and normal tonsillar B cells, expression of the DAT and all five DA receptors (D1–D5) was investigated (for the first time among pure human B cell populations) by RT-PCR. Representative PCR images shown in Fig. 3a were reproducible at different passage number for each cell line. DAT transcripts were evident in most lines: REC-1, derived from a patient with mantle cell lymphoma, was notable in consistently generating a band at least as intense as that from the human DAT transfectant. D2 was present across all lines and in normal B cells (resting or stimulated), whereas D4 was uniformly undetectable. D3 appeared occasionally, whereas detection of D5 was limited to the diffuse large B cell lymphoma line DOHH2 and K1106 derived from a patient with primary mediastinal B cell lymphoma. D1 was widely expressed both among the malignant panel and in normal B cells, although often as a larger transcript than expected and, indeed, generated from the hD1 transfectant (Fig. 3a). Nevertheless, this 700-bp transcript was authenticated on sequencing (as were all PCR products generated).
Fig. 3.
Human B cells variously express mRNA for the DAT, D1-like DA receptors (D1 and D5), or D2-like DA receptors (D2, D3, or D4), but antiproliferative effects of DA are not reversed by pharmacological blockade of these targets. (a) RT-PCR analysis of mRNA expression in positive controls (lane 1, see Materials and Methods), malignant B cell lines (lanes 2–14 and 17–18), LCL (lanes 15 and 16), normal tonsillar B cells quiescent and freshly isolated (lane 19) or after phorbol 12-myristrate 12-acetate plus ionomycin stimulation for 24, 48, or 72,h (lanes 20–22). Water was included in every RT-PCR as negative control with no bands observed (data not shown). β-Actin was run as a loading control for all samples. Images are representative of RT-PCR carried out on three separate RNA preparations. (b) L3055 cells seeded at 5 × 104 per milliliter were preincubated with D2-like DA receptor antagonists (1.5 μM fluphenazine, 3 μM haloperidol, or 2 μM spiperone), the D1-like DA receptor antagonist SCH23390, or DAT antagonists (1 μM GBR12909 or 4 μM mazindol) for 30 min before addition of DA. Cells were incubated for 24 h, and DNA synthesis was assessed as in Fig. 1. Data are presented as percentage of control (mean ± SD from three independent experiments).
To ascertain the contribution (or otherwise) of receptors or transporter to the antiproliferative effects of DA, pharmacological antagonism of dopaminergic targets was undertaken. Initial titration against L3055 cells established the maximal concentration at which antagonists could be applied without toxicity: in each case, this equated to use at levels well above those required to fully antagonize DA at respective structures (19–22). Antagonism of D1-like receptors (D1 and D5) with SCH23390 or D2-like receptors (D2, D3, and D4) with fluphenazine, haloperidol, or spiperone failed to reverse DA’s antiproliferative actions. Pharmacological blockade of the DAT with the high-affinity compounds mazindol or GBR12909 similarly had no effect on DA-dependent cytostasis (Fig. 3b). Inhibiting monoamine oxidases (which convert intracellular DA into oxidative metabolites) with clorgyline, l-deprenyl, or pargyline also failed to affect DA’s antiproliferative actions (data not shown).
Oxidative Stress Constitutes a Primary Mechanism of DA-Induced B Cell Cytostasis.
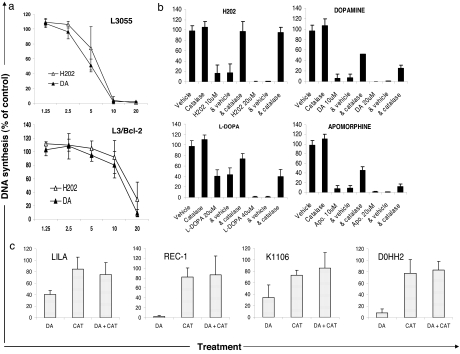
The seeming exclusion of receptors and transporter from delivering DA cytostasis to cycling B cells turned our attention to the catecholamine’s tendency to generate, via autooxidation, reactive oxidation species as a mechanism for the outcomes observed. In initial support of this possibility, we found that L3055 cells (wild-type or bcl-2-transfected) demonstrated a remarkably similar concentration-dependent antiproliferative response to DA and hydrogen peroxide (the latter a metabolite of the former) (Fig. 4a). Catalase, an endogenous enzyme that neutralizes H2O2, was fully effective in reversing hydrogen peroxide’s action against L3055 cells while significantly attenuating the effects of DA, l-DOPA, and apomorphine (Fig. 4b). Catalase also substantively reversed DA cytostasis in a spectrum of malignancies; of the four represented in Fig. 4c it can be noted that reversal was sometimes complete. Residual catalase-unprotected activity of the dopaminergic compounds against, e.g., L3055 cells could be due to additional reactive oxidation species products generated from the inherently unstable catechol ring. These products include quinones and semiquinones, which redox-cycle to generate superoxide; reactive intermediates in the pathway to neuromelanin synthesis may also contribute toxicity (23, 24). Sodium metabisulfite, a general scavenger of H2O2, superoxide, and hydroxyl radicals, did have an impact on DA’s effect on L3055 proliferation as follows: sodium metabisulfite alone, 116 ± 8.5% (of control DNA synthesis); 8 μM DA, 16.1 ± 5.3%; sodium metabisulfite plus DA, 55.3 ± 7.1%. Superoxide dismutase, which neutralizes superoxide radicals, effected a minor reduction in DA’s cytostatic profile (data not shown). Other antioxidants tested but without effect were N-acetyl cysteine, ascorbic acid, reduced glutathione, trolox, and cell-permeable antioxidants (a kind gift of Hazel Szeto, Cornell University, Ithaca, NY) (25).
Fig. 4.
Antiproliferative effects of DA are largely mediated by oxidative stress. (a) Effect on DNA synthesis (as in Fig. 1) of hydrogen peroxide (H2O2) compared with DA against L3055 cells and L3/Bcl-2 transfectants. (b) Cells were preincubated with catalase (1,000 units/ml) for 30 min before addition of H2O2, DA, l-DOPA, or apomorphine as indicated. (c) As in b, but with DA at 10 μM.
Finally, we asked whether a cell’s internal redox profile might influence its sensitivity to DA attack. Although undoubtedly multifactorial, we focused here on thioredoxin (Trx), Bcl-2, and Bax, asking whether among cell lines there was correlation between expression levels and resistance to DA cytostasis, the relative content of each protein being determined by FACS analysis (Table 2, which is published as supporting information on the PNAS web site). As evident from Table 1, no protein could individually account for differing DA sensitivity. However, a significant correlation (P = 0.03) was observed between the product of Trx, Bcl-2, and (reciprocal) Bax levels and relative resistance to 10 μM DA.
Table 1.
Correlation (R2) between resistance to 10 μM DA and levels of protection proteins
| Protein | R2 | P value |
|---|---|---|
| Trx | 0.0771 | n.s. |
| Bcl-2 | 0.0797 | n.s. |
| 1/Bax | 0.0015 | n.s. |
| Bcl-2/Bax | 0.0662 | n.s. |
| Trx/Bax | 0.0264 | n.s. |
| Trx × Bcl-2 | 0.0922 | n.s. |
| (Trx × Bcl-2)/Bax | 0.2729 | 0.03 |
R2 and P values were generated by using the Excel (Microsoft) software toolbox for regression analysis of data on expression levels of Trx, Bcl-2, and Bax (Table 2) vs. the percentage of inhibition of DNA synthesis with 10 μM dopamine (Fig. 1). n.s., not significant.
Discussion
Our data show that rapidly dividing B cells, as exemplified by derived lines from a spectrum of lymphoid malignancy, express dopaminergic pathway constituents and are targeted by DA for cell-cycle arrest, which proceeds to apoptosis, where Bcl-2 levels are low. The antilymphoma outcomes are, however, independent of both DAT and DA receptors, being delivered instead by extracellular oxidation. DA-induced toxicity through the generation of reactive oxidation species is well documented among cell line models of PD (26–28). In general, the concentrations of DA required for affecting neuronal cells via this route appear substantially higher than against the lymphoma lines used here. This observation in itself indicates a heightened sensitivity of proliferating B cells to DA’s actions while suggesting lymphoma cells as valuable adjuncts to investigating mechanisms of catecholamine toxicity.
Although almost all proliferating B cells registered DA-dependent cytostasis, a range of sensitivities was apparent across the populations. Although Bcl-2, Bax, or Trx levels could not individually explain such variation, a composite of their actions may have influenced outcome, with Bcl-2 and Trx contributing resistance and Bax conferring sensitivity. Trx confers survival directly to chronic lymphocytic leukemia cells (29), and by up-regulating Trx peroxidase-1 it protects murine lymphoma cells from “apoptosis caused by H2O2 but not by other agents” (30). Bcl-2 has been implicated in potentiating a cell’s antioxidant capacity with its actions; in turn, it is counteracted by Bax (31). The Bcl-2/Bax ratio is considered by Korsmeyer and colleagues (32) as “a rheostat that regulates an antioxidant pathway.” There are likely additional cellular defense mechanisms contributing resistance to DA/H2O2; nonetheless, factors identified here offer candidates for manipulation to increase therapeutic efficacy via this route of attack.
Bcl-2 was central to whether DA-induced cytostasis was accompanied by apoptosis. Lee and Shacter (33) noted among a panel of BL lines that H2O2-promoted cell death appeared independent of Bcl-2 levels, most likely because the mode of death was deemed nonapoptotic: concentrations of ≥50 μM H2O2 were required to elicit this outcome. For the Jurkat T cell line, <50 μM H2O2 was found to promote caspase activation concomitant with apoptosis, whereas at >200 μM caspases were inhibited and cells became necrotic (34). That Bcl-2 protected L3055 cells from DA-induced caspase activation (and from cell death at concentrations ≤40 μM) is fully consistent with these earlier observations.
While completing the work presented here, we were encouraged to read the findings of Chen et al. (35) whereby ascorbic acid, through extracellular generation of H2O2, was shown to kill cancer cells selectively. Despite offering different vehicles for delivering the toxic hit, there are remarkable parallels between our two studies. In both, the “pro-drug” affected lymphoma cells with “little or no killing of normal lymphocytes and monocytes.” The actions of both vehicles were mimicked by H2O2 and reversed by exogenous catalase. At relatively low concentrations of H2O2 (or pro-drug), death was initially apoptotic, shifting to necrosis/pyknosis upon increased exposure time/concentration. Chen et al. (35) were unable to determine a basis for the selectivity of ascorbate/H2O2 for cancer cells, but a similarly restricted profile has been described both by us here and, significantly, for l-DOPA against stimulated vs. resting lymphocytes and nononcogenic 3T3-fibroblasts (36). Also relevant is the observation that CD45RO+ T cells are killed preferentially by H2O2 (IC50 = 5–10 μM) compared with generally noncycling CD45RA+ counterparts (37). Taken together, the different studies underscore an exquisite, yet unexplained, sensitivity of proliferating lymphocytes, malignant or otherwise, to cellular assault from reactive metabolites of H2O2 and its precursors.
Although the pharmacology essentially excludes both DAT and receptors from DA cytostasis, it remains of interest that B cells and their malignancies should express these components. Frequent detection of D1 and D2 receptor transcripts contrasted with the occasional presence of D3 and D5 and the total absence of D4; DAT was detectable in most lines. We have recently reviewed the literature concerning the presence of DA receptors and DAT on immune cells; previous studies concentrated on T lymphocytes (14, 15). Notably, the consistent presence of the D2 “autoreceptor” sits comfortably with the concept of lymphocytes as an autonomous dopaminergic system whereby tyrosine hydroxylase-dependent DA synthesis provides a means for autocrine regulation, potentially via apoptosis (38–41).
The central question now is whether the observations embodied in this study can be harnessed to formulate a deliverable therapeutic. Apomorphine, an antiparkinsonian drug also used to treat erectile dysfunction, was as effective as DA against model BL cells; nevertheless, concentrations required to achieve successful killing in vitro is >100 times that reached on current dosing in vivo. There is evidence that lymphocytes from PD patients receiving high-dose l-DOPA have increased DA content (42); whether encouraging its release would generate a sufficient local source of extracellular H2O2 to effect apoptosis remains speculative. Of more immediate promise are ongoing developments in relevant drug-delivery systems, e.g., by combining the liposomal packaging of l-DOPA for treating PD (43) with the principle of anti-CD19/CD20-targeted “immunoliposomes” for B lymphoma (44). This consideration applies equally to nonmalignant lymphoproliferative disorders; noncycling B cells are spared the resultant toxic hit. The special case of CNS lymphoma, be it primary or secondary, may particularly benefit from advances in PD treatments where local intracranial delivery of DA or pro-drug becomes a common goal (45).
Materials and Methods
Cells and Reagents.
Origin and karyotypic features of derived lines from patients with B cell malignancy are as detailed elsewhere (11). Stable bcl-2 and bcl-xL transfectants of L3055 cells and subclones containing empty vector are as described (46, 47). LCL were generated by Epstein–Barr virus transformation of peripheral blood B cells (Institute of Cancer Studies, University of Birmingham). PBMC and resting tonsillar B cells were isolated as elsewhere (48). HEK-293 cell transfectants were cultured as previously described (11); HEK-D5 cells were selected on blasticidine S hydrochloride (10 μg/ml).
RPMI medium 1640, DMEM, penicillin/streptomycin, and l-glutamine were from Invitrogen (Paisley, Scotland). Serum supreme was from Cambrex Bioscience (Wokingham, U.K.), FCS was from First Link (Birmingham, U.K.), ionomycin was from Merck Biosciences (Nottingham, U.K.), and phorbol 12-myristate 13-acetate was from Calbiochem (Nottingham, U.K.). Soluble recombinant CD40 ligand was a kind gift from Richard Armitage (Amgen, Seattle, WA). SCH23390 and spiperone were from Tocris Cookson (Avon, U.K.), haloperidol was from Janssen (Buckinghamshire, U.K.), a 100-bp DNA ladder was from Invitrogen (Paisley, Scotland), and DEPC-treated water was from Ambion (Austin, TX). All other reagents were from Sigma–Aldrich (Dorset, U.K.).
Cell Death, Proliferation Assays, and Intracellular Protein Measurements.
DNA synthesis was determined by [3H]thymidine (Amersham, Buckinghamshire, U.K.) incorporation (12). Apoptosis was assessed by flow cytometric analysis via dual staining with PI and the caspase substrate Phiphilux-G1D2 (Oncoimmunin, Gaithersburg, MD) (11). Intracellular Bcl-2 measurement was as previously described (11). For Bax, rabbit polyclonal IgG anti-Bax (1/100; Santa Cruz Biotechnology, Santa Cruz, CA) with unconjugated rabbit IgG (1/100) provided the primary stain with FITC-conjugated goat F(ab)2 anti-rabbit IgG (H+L) (1/100) as secondary. For Trx, 2G11 mAb (1 μg/ml; IMCO, Stockholm, Sweden) controlled with unconjugated mouse IgG1 (1/2,000) was followed with goat anti-mouse FITC (1/50; Dako, Ely, U.K.).
RT-PCR.
RNA extraction (TRIzol reagent, Invitrogen), removal of DNA and protein (RNeasy mini kit, Qiagen, West Sussex, U.K.), and DNA digestion (DNase I reagent, Invitrogen) were carried out according to the manufacturers’ protocol. Reverse transcription was carried out by using SuperScript II (Invitrogen) with primers (synthesized by MWG Biotech, London, U.K.) based on neuronal sequences for the DAT/DA receptors. Details are provided in Tables 3 and 4, which are published as supporting information on the PNAS web site. For RT-PCR of the DAT and DA D1 and D5 receptors, HEK-293 cells stably transfected with human DAT (kindly provided by Bertha Madras, Harvard Medical School, Boston, MA), hD1 (supplied by Mario Tiberi, Ottawa Health Research Institute, Ottawa, Ontario, Canada), or hD5 (Pedro Jose, Georgetown University School of Medicine, Washington, DC) were utilized as positive controls. COS-7 cells transiently transfected with the hD4 receptor were used for the D4 RT-PCR and PBMC for D2 and D3. PCR was carried out with Amplitaq Gold (Applera, Worrington, U.K) and visualized with UV light on 1.2% agarose gels containing ethidium bromide. RT-PCR products were isolated (QIAquick gel extraction kit, Qiagen) according to the manufacturer’s protocol. DNA sequences were verified by MWG Biotech (Milton Keynes, U.K.).
Supplementary Material
Acknowledgments
This work was supported by the Leukaemia Research Fund (U.K.), a University of Birmingham Medical School Studentship, and Swedish Cancer Association Grant 3171 GSD (to A.R.).
Abbreviations
- BL
Burkitt’s lymphoma
- DA
dopamine
- DAT
DA transporter
- l-DOPA
3,4-dihdroxy-l-phenylalanine
- LCL
lymphoblastoid cell lines
- NHL
non-Hodgkin’s lymphoma
- PD
Parkinson’s disease
- PI
propidium iodide
- PBMC
peripheral blood mononuclear cell
- Trx
thioredoxin.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Ansell S., Armitage J. Mayo Clin. Proc. 2005;80:1087–1097. doi: 10.4065/80.8.1087. [DOI] [PubMed] [Google Scholar]
- 2.Traulle C., Coiffier B. Future Oncol. 2005;1:297–306. doi: 10.1517/14796694.1.3.297. [DOI] [PubMed] [Google Scholar]
- 3.Maher E., Fine H. Semin. Oncol. 1999;26:346–356. [PubMed] [Google Scholar]
- 4.Navarro W. H., Kaplan L. D. Blood. 2006;107:13–20. doi: 10.1182/blood-2004-11-4278. [DOI] [PubMed] [Google Scholar]
- 5.Spina M., Simonelli C., Talamini R., Tirelli U. J. Clin. Oncol. 2005;23:8132–8133. doi: 10.1200/JCO.2005.02.9561. [DOI] [PubMed] [Google Scholar]
- 6.Hesseling P., Broadhead R., Mansvelt E., Louw M., Wessels G., Borgstein E., Schneider J., Molyneux E. Pediatr. Blood Cancer. 2005;44:245–250. doi: 10.1002/pbc.20254. [DOI] [PubMed] [Google Scholar]
- 7.Lavu E., Morewaya J., Maraka R., Kiromat M., Ripa P., Vince J. Ann. Trop. Paediatr. 2005;25:191–197. doi: 10.1179/146532805X58120. [DOI] [PubMed] [Google Scholar]
- 8.Meinl E., Krumbholz M., Hohlfeld R. Ann. Neurol. 2006;59:880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell P., Wang X., Leon-Ponte M., Griffiths C., Pingle S., Ahern G. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub R. Trends Pharmacol. Sci. 2004;25:640–646. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Meredith E., Holder M., Chamba A., Challa A., Drake-Lee A., Bunce C., Drayson M., Pilkington G., Blakely R., Dyer M. J., et al. FASEB J. 2005;19:1187–1189. doi: 10.1096/fj.04-3477fje. [DOI] [PubMed] [Google Scholar]
- 12.Serafeim A., Grafton G., Chamba A., Gregory C. D., Blakely R. D., Bowery N. G., Barnes N. M., Gordon J. Blood. 2002;99:2545–2553. doi: 10.1182/blood.v99.7.2545. [DOI] [PubMed] [Google Scholar]
- 13.Serafeim A., Holder M. J., Grafton G., Chamba A., Drayson M. T., Luong Q. T., Bunce C. M., Gregory C. D., Barnes N. M., Gordon J. Blood. 2003;101:3212–3219. doi: 10.1182/blood-2002-07-2044. [DOI] [PubMed] [Google Scholar]
- 14.Gordon J., Barnes N. Trends Immunol. 2003;24:438–443. doi: 10.1016/s1471-4906(03)00176-5. [DOI] [PubMed] [Google Scholar]
- 15.Meredith E., Chamba A., Holder M., Barnes N., Gordon J. Immunology. 2005;115:289–295. doi: 10.1111/j.1365-2567.2005.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston T., Fox S., Brotchie J. Expert Opin. Drug Delivery. 2005;2:1059–1073. doi: 10.1517/17425247.2.6.1059. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Mason D., Johnson G., Abbot S., Gregory C., Hardie D., Gordon J., MacLennan I. Eur. J. Immunol. 1991;21:1905–1910. doi: 10.1002/eji.1830210819. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki H., Hata H., Asou N., Suzushima H., Akahoshi Y., Yoshida M., Nagakura S., Ishii T., Sanada I., Takatsuki K. Acta. Haematol. 1990;84:156–161. doi: 10.1159/000205054. [DOI] [PubMed] [Google Scholar]
- 19.Andersen P. Eur. J. Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- 20.Civelli O., Zhou Q. Encyclopedia of Life Sciences. Vol. 2006. Chichester, U.K.: Wiley; 2001. [Google Scholar]
- 21.Heikkila R., Babington R., Houlihan W. Eur. J. Pharmacol. 1981;71:277–286. doi: 10.1016/0014-2999(81)90030-3. [DOI] [PubMed] [Google Scholar]
- 22.Roth B., Lopez E., Patel S., Kroeze W. Neuroscientist. 2000;6:252–262. [Google Scholar]
- 23.Slominski A., Paus R., Mihm M. Anticancer Res. 1998;18:3709–3715. [PubMed] [Google Scholar]
- 24.Slominski A., Tobin D., Shibahara S., Wortsman J. Physiol. Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 25.Zhao K., Zhao G., Wu D., Soong Y., Birk A., Schiller P., Szeto H. J. Biol. Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 26.Clement M., Long L. H., Ramalingam J., Halliwell B. J. Neurochem. 2002;81:414–421. doi: 10.1046/j.1471-4159.2002.00802.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim J., Kwon K., Yoon H., Lee S., Rhee S. Arch. Biochem. Biophys. 2002;397:414–423. doi: 10.1006/abbi.2001.2691. [DOI] [PubMed] [Google Scholar]
- 28.Lotharius J., O’Malley K. J. Biol. Chem. 2000;275:38581–38588. doi: 10.1074/jbc.M005385200. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson J., Soderberg O., Nilsson K., Rosen A. Blood. 2000;95:1420–1426. [PubMed] [Google Scholar]
- 30.Berggren M., Husbeck B., Samulitis B., Baker A., Gallegos A., Powis G. Arch. Biochem. Biophys. 2001;392:103–109. doi: 10.1006/abbi.2001.2435. [DOI] [PubMed] [Google Scholar]
- 31.Jang J., Surh Y. Biochem. Pharmacol. 2003;66:1371–1379. doi: 10.1016/s0006-2952(03)00487-8. [DOI] [PubMed] [Google Scholar]
- 32.Korsmeyer S., Shutter J., Veis D., Merry D., Oltvai Z. Semin. Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- 33.Lee Y., Shacter E. Blood. 1997;89:4480–4492. [PubMed] [Google Scholar]
- 34.Hampton M., Orrenius S. FEBS Lett. 1997;414:552–556. doi: 10.1016/s0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q., Espey M. G., Krishna M. C., Mitchell J. B., Corpe C. P., Buettner G. R., Shacter E., Levine M. Proc. Natl. Acad. Sci. USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slominski A., Goodman-Snitkoff G. Anticancer Res. 1992;12:753–756. [PubMed] [Google Scholar]
- 37.Takahashi A., Hanson M., Norell H., Havelka A., Kono K., Malmberg K., Kiessling R. J. Immunol. 2005;174:6080–6087. doi: 10.4049/jimmunol.174.10.6080. [DOI] [PubMed] [Google Scholar]
- 38.Bergquist J., Tarkowski A., Ewing A., Ekman R. Immunol. Today. 1998;19:562–567. doi: 10.1016/s0167-5699(98)01367-x. [DOI] [PubMed] [Google Scholar]
- 39.Bergquist J., Josefsson E., Tarkowski A., Ekman R., Ewing A. Electrophoresis. 1997;18:1760–1766. doi: 10.1002/elps.1150181009. [DOI] [PubMed] [Google Scholar]
- 40.Bergquist J., Ohlsson B., Tarkowski A. Neuroimmunomodulation. 2000;917:281–289. doi: 10.1111/j.1749-6632.2000.tb05394.x. [DOI] [PubMed] [Google Scholar]
- 41.Bergquist J., Tarkowski A., Ekman R., Ewing A. Proc. Natl. Acad. Sci. USA. 1994;91:12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajda C., Dibo G., Vecsei L., Bergquist J. Neuroimmunomodulation. 2005;12:81–84. doi: 10.1159/000083579. [DOI] [PubMed] [Google Scholar]
- 43.Di Stefano A., Carafa M., Sozio P., Pinnen F., Braghiroli D., Orlando G., Cannazza G., Ricciutelli M., Marianecci C., Santucci E. J. Control Release. 2004;99:293–300. doi: 10.1016/j.jconrel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Sapra P., Allen T. Clin. Cancer Res. 2004;10:2530–2537. doi: 10.1158/1078-0432.ccr-03-0376. [DOI] [PubMed] [Google Scholar]
- 45.Jain N., Rana A., Jain S. Drug Dev. Ind. Pharm. 1998;24:671–675. doi: 10.3109/03639049809082370. [DOI] [PubMed] [Google Scholar]
- 46.Milner A., Johnson G., Gregory C. Int. J. Cancer. 1992;52:636–644. doi: 10.1002/ijc.2910520424. [DOI] [PubMed] [Google Scholar]
- 47.Wang H., Grand R., Milner A., Armitage R., Gordon J., Gregory C. Oncogene. 1996;13:373–379. [PubMed] [Google Scholar]
- 48.McCloskey N., Pound J., Holder M., Williams J., Roberts L., Lord J., Gordon J. Eur. J. Immunol. 1999;29:3236–3244. doi: 10.1002/(SICI)1521-4141(199910)29:10<3236::AID-IMMU3236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.