Abstract
A number of electrophysiological experiments have shown that odor exposure alone, unaccompanied by behavioral training, changes the response patterns of neurons in the olfactory bulb. As a consequence of these changes, across mitral cells in the olfactory bulb, individual odors should be better discriminated because of previous exposure. We have previously shown that a daily 2-h exposure to odorants during 2 weeks enhances rats' ability to discriminate between chemically similar odorants. Here, we first show that the perception of test odorants is only modulated by enrichment with odorants that activate at least partially overlapping regions of the olfactory bulb. Second, we show that a broad activation of olfactory bulb neurons by daily local infusion of NMDA into both olfactory bulbs enhances the discrimination between chemically related odorants in a manner similar to the effect of daily exposure to odorants. Computational modeling of the olfactory bulb suggests that activity-dependent plasticity in the olfactory bulb can support the observed modulation in olfactory discrimination capability by enhancing contrast and synchronization in the olfactory bulb. Last, we show that blockade of NMDA receptors in the olfactory bulb impairs the effects of daily enrichment, suggesting that NMDA-dependent plasticity is involved in the changes in olfactory processing observed here.
Keywords: discrimination, enrichment, plasticity
Behavioral enrichment is thought to be a major modulator of animals' ability to learn behavioral tasks. It has been shown many times that an enriched environment during development dramatically increases animals' learning and memory abilities during adult life (1, 2). Enrichment during adult life can counteract learning disabilities due to absence of NMDA receptors in genetically modified mice (3), as well as the decrease in learning capabilities seen in older rodents (4, 5). Most studies investigating the beneficial effects of enriched housing environments focus on complex learning tasks such as the Morris water maze (5–7) or the Hebb–Williams maze (8) that have been linked to hippocampal function (9–11). In contrast to previous studies, we here focus on sensory perception, a predeterminant of all learning tasks in animals. Using olfactory perception in rats, we have recently shown for the first time that sensory enrichment during a relatively short period improves animals' sensory discrimination capabilities in an odor-unspecific manner (12). Here, we show that the modulation of olfactory discrimination abilities due to daily enrichment with odorants arises in the first olfactory sensory structure, the olfactory bulb (OB). First, we show that enrichment only affects the perception of those odorants that activate at least partially overlapping regions of the OB, suggesting that nonspecific activation of OB neurons suffices to produce lasting changes in perception and that spatial specificity of odor responses is of importance in this process. Second, we show that unspecific activation of OB neurons via local injections of NMDA produces changes in odor perception similar to those observed after daily enrichment with odorants. Third, using a computational model of OB odor processing, we propose that activation of OB neurons produces widespread changes in OB inhibitory processes that can underlie the observed increase in odor discrimination. Last, we show that local blockade of NMDA receptors blocks the effects of daily odor enrichment.
Results
We have previously shown a relatively nonspecific improvement of odor discrimination in response to olfactory enrichment: after daily exposure to a single odor pair, rats' discrimination ability of all odor pairs tested in that experiment were improved. Here, we further investigate the localization and mechanisms of this improvement.
Experiment 1: For Perceptual Modulation to Occur, Enrichment and Test Odorants Must Activate Partially Overlapping Regions of the Olfactory Bulb.
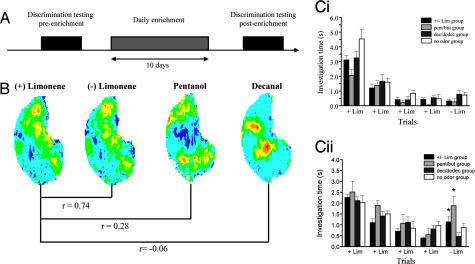
In this experiment, we used a habituation test to assess spontaneous olfactory discrimination between a pair of chemically and perceptually very similar odorants, + and − limonene (+/− lim) (Table 1) (13) as a function of the overlap between these test odorants and the odorants used during the enrichment period. Discrimination was assessed before and after a 10-day enrichment period (Fig. 1A). To evaluate how nonspecific the effects of daily odor exposure are, we used four experimental groups, each receiving enrichment with odorants exhibiting various degrees of response overlap with + and − lim (Fig. 1 A and B). Rats were exposed +/− lim, pentanol/butanol (pent/but), decanol/dodecanol (dec/dodec) or no odor. + lim partially overlaps in its activation of glomeruli with pent (r = 0.28) (B. Johnson, personal communication), whereas it does not overlap with dec (r = −0.06) (B. Johnson, personal communication). We first confirmed that before enrichment, the two enantiomers of lim were not discriminated by the rats [P > 0.05 (Fisher) for all groups; Fig. 1Ci]. An overall ANOVA with experimental groups (+/− lim, pent/but, dec/dodec, and no odor), test (preenrichment test and postenrichment test), and trial number as main effects showed no significant effect of experimental group [F(3, 220) = 2.575; P = 0.055] or of test [F(1, 220) = 0.576; P = 0.44] but an effect of trial number [F(4, 220) = 85.77; P < 0.001]. An interaction between experimental group, test, and trial number was observed [F(12, 220) = 1.824; P < 0.05], indicating that the observed effect of trial number depends on both the experimental groups and the test. Significant habituation for all groups in pre- and posttesting conditions was observed (difference between the first and the last habituation trial; P < 0.005 in all cases). As expected, control rats exposed to the mineral oil carrier only daily for 1 h during 10 days showed no improvement of discrimination (P = 0.804, Fisher; Fig. 1Cii). In contrast, a significant increase in investigation time during the test trial was observed in +/− lim-enriched group (P = 0.004; Fig. 1Cii) as well as in the pent/but-enriched group (P = 0.027; Fig. 1Cii). Interestingly, the discrimination between the enantiomers of lim was not improved after enrichment with odors that do not activate overlapping OB regions (dec/dodec; P = 0.228; Fig. 1Cii).
Table 1.
Odors used for habituation experiments and corresponding dilutions
| Odor pair | Odorant |
|
|---|---|---|
| Ohab (%) | Otest (%) | |
| 1 | + lim (0.2) | − lim (0.2) |
| 2 | + terp (6.63) | − terp (6.63) |
Ohab, habituation odor; Otest, test odor.
Fig. 1.
Perception of odorants is only modulated by enrichment with odorants that activate at least partially overlapping regions of the OB. (A) Time course of the experiment. (B) Maps of 2-dexoxyglucose (2-DG) uptake across the entire glomerular layer in rats exposed to + lim, pent, and dec. Overlaps between + lim and pent (pairwise correlation coefficient, r = 0.28) are more important than overlaps between + lim and dec (pairwise correlation coefficient, r = −0.06). Courtesy of B. Johnson and M. Leon (http://leonlab.bio.uci.edu). (C) Behavioral habituation and discrimination before (Ci) and after (Cii) enrichment. The two enantiomers of lim are confused before the enrichment period, whereas after enrichment with +/− lim or pent/but, they are discriminated. Enrichment with dec/dodec has the same effect as no odor, indicating that the discrimination is improved when the enrichment is done with a partially overlapped odor. Asterisks indicate a significant difference (P < 0.05) in response magnitude between trials 4 and 5.
These results show that although enrichment is relatively nonspecific to the test odors, there needs to be at least a partial overlap between the odor-responsive regions of the OB of enrichment and test odors for modulation to occur. To show that this effect is long-lasting, we tested rats at 2, 20, and 40 days after the end of the enrichment phase. We found that the effects of daily exposure to odorants was still present after 20 but not 40 days. Significant habituation was observed for all tests (difference between the first and the fourth habituation trial; P < 0.005 in all cases), and before the enrichment phase, rats did not discriminate between +/− lim (P > 0.05 in all cases). After daily exposure to the two enantiomers of lim, the response to − lim was significantly higher than the response to + lim 2 days (P = 0.004) and 20 days (P = 0.003) but not 40 days after the enrichment period ended (P = 0.429).
Experiment 2: Broad, Nonspecific Activation of the OB Modulates Odor Perception.
To gain an understanding of the mechanisms underlying the enrichment-induced modulation of olfactory perception, we hypothesized that daily activation of the OB due to odor exposure may be responsible for changes in the OB. To test this hypothesis, we induced a broad activation of OB neurons by daily local infusion of NMDA into both OBs. Cannulated rats with daily infusions of NMDA but no odor exposure were compared with a group of rats exposed to +/− lim for 1 h daily and injected with saline solution into the OB (Fig. 1A). We did not observe any changes in behavior after NMDA injection.
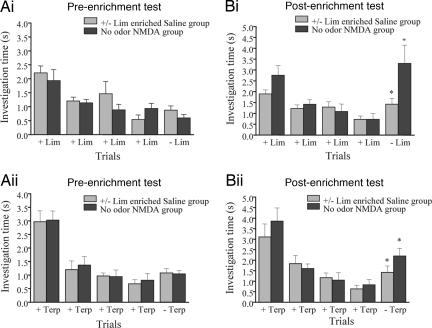
An overall ANOVA using experimental group (saline + lim and NMDA + no odor), test (pre- and postenrichment), test odor [+/− lim and +/− terp (terpinen-4-ol)], and trial number (1 to 5) as main effects yielded no significant effect of group [F(2, 278) = 1.847; P > 0.1] or of odor [F(1, 278) = 3.188; P > 0.05], indicating that both groups performed similarly in response to both odorants. However, a significant effect of test [F(1, 278) = 9.428; P < 0.05] as well as of trial number [F(4, 278) = 30.376; P < 0.001] was found, showing that rats behaved significantly differently during post- and pretests and that investigation times changed as a function of trial number. In addition, a significant interaction was found between test and trial number [F(4, 278) = 2.732; P < 0.05] suggesting that between pre- and posttesting, rats differed in their responses over habituation trials.
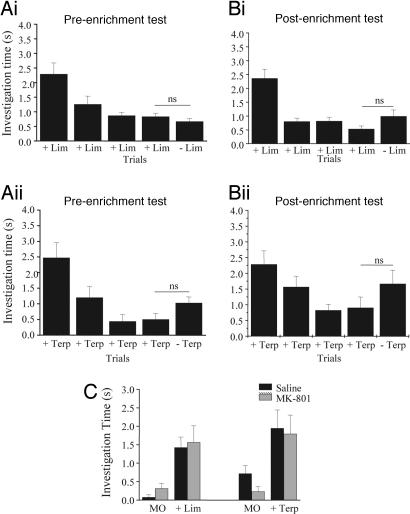
Post hoc tests (Fisher) showed significant habituation for all groups (difference between the first and the fourth habituation trial; P < 0.005 in all cases; Fig. 2). Before enrichment, no experimental group discriminated between +/− lim (Fig. 2Ai) or +/− terp (Fig. 2Aii) (P > 0.05 for all groups). After +/− lim enrichment accompanied with saline injection into the OB, the response to − lim was significantly higher than the response to + lim (P = 0.02; Fig. 2Bi). Similarly, in that group, the response to − terp was significantly higher than the response to + terp (P = 0.04; Fig. 2Bii). After daily NMDA injections in the OB for 10 days but no odor exposure, rats also discriminated between + lim and − lim (P = 0.004; Fig. 2Ai), as well as between + terp and − terp (P = 0.04; Fig. 2Bii).
Fig. 2.
Effect of nonspecific activation of the OB using local infusions of NMDA. (A) The two enantiomers of lim (Ai) and terp (Aii) were not discriminated before enrichment. (B) After the enrichment period, both groups discriminated +/− lim (Bi) and +/− terp (Bii).
These results show that broad, nonspecific activation of the OB suffices to produce durable changes in OB processing of odorants.
Experiment 3: Computational Model.
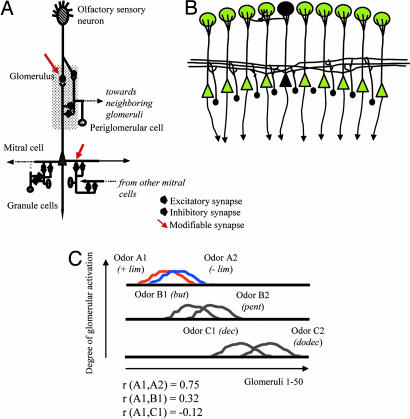
To test how known characteristics of the OB network could underlie these behavioral observations, we used a computational model of the OB. Here, we tested how modulation of synaptic strength in response to odor activation could result in changes of responses to other, overlapping odorants. Three chemically similar pairs of odorants were simulated; the overlap between the two members of each pair was high (r = 0.74 for simulated enantiomers of lim for example) as described experimentally (Fig. 1B) (13, 14). Similarly to the experimental odors, pairs of test odorants were chosen such that they either activated partially overlapping regions [lim and pent (r = 0.28) in Fig. 1B; odors A1 and B1 (r = 0.32) in Fig. 3C] or completely nonoverlapping regions [lim and dec (r = −0.06) in Fig. 1B; odors A1 and C1 (r = −0.12) in Fig. 3C] at the input of the OB model.
Fig. 3.
Computational modeling. (A) Schematic illustration of the OB model. A total of 50 OSNs, each representing the population response of OSNs with similar odorant-binding affinities, each project to a single glomerulus in which they make excitatory synapses onto mitral cells and periglomerular cells. Each mitral cell represents the average activity of the 10–15 mitral cells with primary dendrites in a given glomerulus. Mitral and periglomerular cells have reciprocal dendrodendritic synaptic interactions in which mitral cells are excitatory and periglomerular cells are inhibitory. In addition to these interglomerular interactions, periglomerular cells also interact with mitral cells in a small (three to four) number of surrounding glomeruli. Mitral cell secondary dendrites excite granule cells in a wide area of the model, and each mitral cell forms excitatory synaptic connections with ≈50% of the model granule cells. Granule cells in turn inhibit mitral cells via dendrodendritic inhibitory synapses. Modifiable excitatory synapses (arrows) were implemented between OSNs and mitral cells as well as between mitral and granule cells, as described in refs. 15–17. During activity-induced plasticity, the synaptic strengths of these synapses were updated by means of a classical hebbian learning rule in which the change in synaptic strength depends on pre- and postsynaptic activity (see Methods for details). (B) Because each mitral cell (black triangle) excites a large number of granule cells in the model (black circles), odor activation of a single mitral cell spreads activation to a large fraction of granule cells, leading to changes in odor processing that affect mitral cell activation by other odorants. (C) Simulated odorants. In the model, all odorants activate a subset (25–30%) of OSNs to various degrees. Odorants were chosen in such a manner that the OSN patterns overlapped to the same degree as experimental odorants (see Fig. 1B). The graph shows the average activation of each glomerulus during the time of stimulus application in response to the six odorants used in the simulations. For ease of comparison, each pair of test odorants is shown on a separate axis. For comparison with experimental data from Fig. 1, the correlation values between odor A1, B1, and C1 are given on this graph.
These simulated odorants differentially activated 50 olfactory sensory neurons (OSNs) in the model (Fig. 3A), each representing a large population of sensory neurons with similar odor-binding properties and activating the OB model as detailed in Methods and in Fig. 3A. As a result of the widespread interactions between mitral and granule cells, activation spreads from a single mitral cell to a large portion of the granule cells in the model (Fig. 3B). Mitral cell spiking responses at the output of the OB model are the result of intrabulbar computations. Because of the long-lasting changes in odor perception after daily exposure to odorants, we hypothesized that an activity-dependent synaptic plasticity at the level of two synapses could produce changes in OB processing that affect a wide array of odors. We therefore implemented a simple activity-dependent hebbian learning rule at the synapse between OSNs and mitral cells and between mitral and granule cells; both of these synapse types have been shown to undergo activity-dependent plasticity experimentally (15–17).
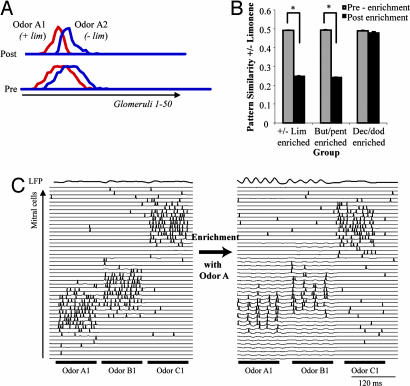
We tested how activity-dependent plasticity in response to activation with simulated +/− lim (odors A1 and A2), pent/but (odors B1 and B2), and dec/dodec (odors C1 and C2) affected the subsequent processing of the enantiomers of lim. These simulations followed the protocol of experiment 1 in which the effect of enrichment with +/− lim, pent/but, or dec/dodec on the perceptual discrimination between + and − lim was tested. We calculated the normalized scalar product between mitral cell activation vectors in response to odors A1 and A2 before odor-induced plasticity [Fig. 4 A (pre) and B (before-enrichment)] and after plasticity was induced in response to odors A1/A2, B1/B2, or C1/C2. Fig. 4A shows the average mitral cell response activations in response to a 200-ms stimulation with odors A1 and A2 before (pre) and after (post) plasticity induced with odors A1 and A2. Comparison of the two graphs shows that after the plasticity phase, the population of mitral cells responding to odors A1 and A2 are more distinct. Average overlaps between mitral cell responses to A1 and A2 were decreased significantly after a plasticity phase induced by stimulating the model with odors A1 and A2 (Fig. 4B, +/− lim-enriched) or with odors B1 and B2 (Fig. 4B, but/pent-enriched) but not after plasticity was induced by stimulating the model with odorants C1 and C2 (Fig. 4B, dec/dodec-enriched). These results replicate the behavioral results from experiment 1 in which the spontaneous discrimination between the enantiomers of lim increased after enrichment with +/− lim or pent/but but not dec/dodec. The hypothesis that the difference arises from the degree of overlap between test odors and enrichment odorants was thus successfully tested in the model. Fig. 4C illustrates how odor-induced plasticity with one odorant affects subsequent processing of that and other odorants. In Fig. 4C, we first stimulated the model with odors A1, B1, and C1 for 100 ms each. One can easily see that mitral cell responses to odors A1 and B1 overlap substantially (because of the overlap in receptor cell activation), but responses to A1 and C1 do not overlap. The model was then submitted to a phase of activity-induced plasticity meant to mimic the daily exposure to odors A1 and A2 and stimulated again with the same three odorants. Because of the activity-induced modulation of synaptic strength between OSNs and mitral cells as well as between mitral and granule cells, two changes in OB response to odorants can be observed: (i) increased oscillatory activity and synchronization of spikes in a broad area of the OB model, and (ii) decreased number of mitral cells responding with increased firing rates to odor stimulation. Both of these changes in OB responses can potentially contribute to the behaviorally observed modulation of odor perception (18–23).
Fig. 4.
Simulation results. (A) Average mitral cell activation (numbers of action potentials fired during the time of odor application) across all 50 glomeruli in response to odors A1 and A2 are depicted before (pre) and after (post) activity-induced synaptic plasticity in response to odorants A1 + A2. One can see that the overlap between average mitral cell responses is reduced after the plasticity period. (B) Results from simulated olfactory enrichment experiments. The graphs show the average overlap (normalized scalar product) between network responses to odors A1 and A2 (representing +/− lim) before and after enrichment with A1 + A2 (+/− lim-enriched), B1 + B2 (but/pent-enriched), and C1 + C2 (dec/dodec-enriched). Each graph shows the average and standard deviation of 50 independent simulations. The overlap between responses to odors A1 and A2 is significantly decreased after a plasticity phase in response to odorants A1 + A2 or B1 + B2 but not C1 + C2. (C) Mitral cell responses to odorants are shown as a function of time. The subset of mitral cells responding to odors A1, B1, and C1 are shown. The network was first stimulated with odors A1, B1, and C1 for 100 ms each. It was then submitted to a phase of synaptic plasticity mimicking daily odor exposure with odors A1 and A2. Subsequently, the network was again activated with odors A1, B1, and C1 for 100 ms each. Because of the modulation of OB synapses in response to odorants A1 and A2, both the oscillatory response and the mitral cell responses to odorants A1 and B1, but not C1, are changed.
Experiment 4: Enrichment-Induced Modulation of Perception Is NMDA-Dependent.
To test the computational hypothesis about the role of NMDA-dependent plasticity in olfactory enrichment, we injected MK-801 into both OBs before the daily exposure to odorants and compared the rats' discrimination capabilities before and after the enrichment period. Overall ANOVA using experimental group (saline and MK-801, both enriched with lim), test (pre and post), odor (+/− lim and +/− terp), and trial number (1 to 5) as main effects showed a significant effect of group [F(1. 297) = 4.00; P = 0.046] but not of odor [F(1, 297) = 3.044; P = 0.82]. Post hoc tests (Fisher) showed significant habituation for all groups (difference between the first and the fourth habituation trial; P < 0.001 in all cases) (Fig. 5 A and B). Before enrichment, rats did not discriminate between +/− lim and +/− terp (P > 0.05; Fig. 5 Ai and Aii). After MK-801 injections in the OB followed by a 1-h exposure to +/− lim, rats did not discriminate the enantiomers of lim (P = 0.616; Fig. 5Bi) or terp (P = 0.141; Fig. 5Bii). These results show that blockade of NMDA receptors during the daily enrichment with odorants blocks the effect of odor enrichment, as predicted by the computational model.
Fig. 5.
Blockade of NMDA receptors before daily odor exposure blocks the effect of enrichment on odor discrimination. (A) Preenrichment. In these graphs, results from a group of rats in which MK-801 was injected before the daily odor exposure to +/− lim are shown. The two enantiomers of lim (Ai) and terp (Aii) were not discriminated before enrichment. (B) Postenrichment. After the enrichment period, rats could not discriminate between the enantiomers of lim (Bi) or terp (Bii), showing that blockade of OB NMDA receptors blocks the effects of daily odor exposure. (C) OB blockade of NMDA receptors with MK-801 at the concentrations used in these experiments does not impair the detection of lim and terp. Saline-injected control rats and MK-801-injected rats were first habituated to mineral oil during four trials and then exposed to either lim or terp during the fifth trial. Both groups of rats responded significantly more to the test odor during the last trial than to the mineral oil in the previous trial.
We performed additional tests to ensure that rats injected with MK-801 at the dosage used here were still capable of odor perception. Ten implanted rats were used for these tests. A test session consisted of four 50-s presentations of plain mineral oil followed by one 50-s odor presentation. If the odorant (+ lim or + terp) is detected, it will elicit an increased investigation response by the rat. ANOVA with group (MK-801 and saline), odors (+ lim and + terp), and trial as main effects showed no effect of groups [F(1, 95) = 0.191; P > 0.5], no effect of odor [F(1, 93) = 0.086; P = 0.086], and no interactions. Both groups discriminated between the mineral oil and odors (saline/lim, P < 0.001; saline/terp, P = 0.008; MK/lim, P = 0.004; MK-801/terp, P = 0.001) (Fig. 5B). These results clearly show that the lack of effect of odor enrichment observed in the group that received NMDA antagonist injections before the daily exposure to odorants is not due to a lack of odor perception because of NMDA receptor blockade.
Discussion
We have previously shown that sensory enrichment during a relatively short period (i.e., 10 days) improves olfactory discrimination capabilities in rats (12). These behavioral results are in agreement with studies in other systems that have demonstrated that enrichment improved spatial learning performance in rats (24, 25), memory for fear-conditioning learning (26), and recognition memory (3) or had a profound impact on synaptic function and plasticity in the olfactory cortex (27). Here, we report that enrichment only affects the perception of odorants that activate at least partially overlapping regions of the OB, suggesting that nonspecific activation of OB suffices to change perception. Furthermore, nonspecific activation of the OB via daily local injections of NMDA does indeed improved rats' discrimination capabilities in the same manner as daily odor enrichment. Taken together, these results show that activation of the OB alone, in the absence of motivated odor investigation, is sufficient to lead to long-lasting changes in olfactory perception. In agreement with data showing that changes in OB neural responses (28–30) and in OB neurogenesis occur because of olfactory enrichment (31), we clearly show that manipulation of the OB network is sufficient to produce long-lasting perceptual changes. Both the manipulations performed in our model, i.e., synaptic plasticity within the existing inhibitory network or changes in the structure of this inhibitory network, due for example to changes in neurogenesis of inhibitory interneurons (not explicitly modeled here), could be at the basis of the observed changes in olfactory perception.
The computational modeling presented here shows that (i) the modulation of OB synapses known to exhibit activity-dependent plasticity (15–17) suffices to produce changes in OB processing that can underlie the perceptual changes reported here and (ii) that OB architecture and its widespread dendritic interactions can explain the observed relatively nonspecific effects of olfactory enrichment. The model shows that activation with a single chemical can produce changes in a large area of the OB and that these would effect the processing of other odorants, as long as partially overlapping areas of the OB are activated. Behavioral and modeling results clearly show that spatial specificity of activation patterns in response to odor stimulation plays an important role in experience-induced changes in odor processing.
Our analysis provides evidence suggesting an important role of NMDA receptor activity in the OB for discrimination learning. Cellular mechanisms for discrimination are often attributed to the inhibitory circuitry of the OB (refs. 20 and 32–35; for review, see refs. 36–38). Lateral inhibitory circuits were postulated to mediate contrast enhancement (33). Such contrast enhancement may rest in large part on the particular properties of dendrodendritic synapses between the principal output neurons (mitral cells) and local inhibitory neurons (granule cells) of the OB. Fletcher and Wilson (39) have shown that even in the anesthetized rat, prolonged exposure to an odorant can fine-tune the receptive field of mitral cells; the authors interpreted those results as due to an increase in inhibition, as suggested by our computational model. NMDA receptors are critical for the generation of dendrodendritic inhibition (40). Indeed, NMDA antagonists reduce granule cell-mediated inhibition of mitral cells (41) and retard learning of an olfactory discrimination task (42). The modeling results presented here clearly show how the relatively nonspecific effect of odor enrichment arises from OB properties: because of the widespread interaction between mitral and granule cells in the OB, activity-induced plasticity in response to one odorant affects the subsequent processing of odorants that activate partially overlapping regions of the OB. In contrast, the processing of odorants that activate completely nonoverlapping areas is not affected, both with respect to an increase in oscillatory activity and individual mitral cell responses (Fig. 5C). Taking into account the impairment of enrichment improved discrimination after MK-801 injections, we conclude that NMDA receptor activity in the OB is essential for the improvement of olfactory discrimination learning due to enrichment.
Methods
Subjects.
The eight male Sprague–Dawley rats (250–300 g) used for this study were obtained from Charles River Laboratories (Wilmington, MA). Rats were kept on a 12 h light/12 h dark cycle and allowed access to food and water ad libitum. All procedures were performed under the auspices of a protocol approved by the Cornell University Institutional Animal Care and Use Committee.
Enrichment.
For olfactory enrichment, swabs containing 100 μl of pure odor were placed in two tea balls hanging from the cover of standard breeding cage (12, 31) daily for 1 h during 10 days (Fig. 1A). Each of the two tea balls contained a different odor (+ and − lim, pent and but, or dec and dodec). For the control group, rats were housed under the same conditions except that the two tea balls contained only mineral oil.
Olfactory Habituation.
Odors.
For discrimination testing, two different odor pairs were used, each composed of two odorants (habituating odor, Ohab, and test odor, Otest; see Table 1). For both odor pairs, odor Ohab and Otest were chosen to be highly similar to each other (13, 43). The odorants were diluted in mineral oil proportionally to their vapor pressure, as detailed in refs. 43 and 44.
Behavioral testing.
All habituation experiments took place in the home cage of the test animals under a red light. Odors were presented by placing 60 μl of the odor stimulus onto a filter paper (No. 1; Whatman, Florham Park, NJ). The filter paper was put into a weighing dish that was put on top of the wire cage cover. Each rat was tested twice with two different odor sets. A test session consisted of one 50-s presentation of plain mineral oil, then four 50-s odor presentations of the Ohab at 5-min intervals, followed by one 50-s presentation of the Otest. All odor sets were encoded so that the experimenter was unaware of the identity of each odor. The amount of time that the rat investigated the odorant was recorded during all trials. Investigation was defined as active sniffing within 1 cm of the odor.
Cannulation.
Rats were anesthetized with an intramuscular mixture injection of 80 mg/kg ketamine/6 mg/kg xylazine and were secured in the stereotaxic instrument (Narishige Scientific Instrument, Tokyo, Japan). Cannulae (22 gauges; Plastics One, Roanoke, VA) were inserted bilaterally for infusions at the following coordinates from bregma: anteroposterior, −8; mediolateral, ±1.5; dorsoventral, −4.5. The guide cannulae, positioned 1 mm dorsal to the infusion site, and the infusion cannulae, positioned extending 1 mm ventral to the end of the guide cannulae, were secured with screws and dental cement. Dummy cannulae were then placed into the guide cannulae to prevent blockage or infection. After surgery, the rats were allowed to recover for 14 days. Preenrichment and postenrichment tests occurred 2 and 4 weeks, respectively, after the surgery.
Intracerebral Drug Administration.
At the time of infusion, two infusion cannulae were fitted into the guide cannulae and NMDA (15 μg per OB), MK801 (15 μg per OB), or saline infusions were performed by using two 10-μl syringes (Hamilton, Reno, NV) attached to the cannulae with a polyethylene tube. The tip of the infusion cannula protruded 1.0 mm beyond the guide cannula and was aimed at the subventricular zone of the OB. Drugs were delivered bilaterally. The drug was administered in a volume of 6 μl per side at a rate of 2 μl/min for 3 min. The infusion cannula remained in place 1 min after the infusion. The infusion volume used was determined by using 2% pontamine sky blue dye solution in saline, showing that a 6-μl infusion distributes adequately through the OB without appreciably invading other neural structures.
Data Analysis.
All data analyses on time spent sniffing during odor presentation trials were performed with SPSS statistical software (SPSS, Chicago, IL). Only rats that investigated odor A for at least 1 s during its first presentation were included in the analysis. After ANOVA testing for the main effects of treatment group, test (pre or post), and trial number, post hoc pairwise comparisons (Fisher) were performed to determine (i) whether a rat's investigation time during the last habituation trial was significantly lower than during the first habituation trial (habituation) and (ii) whether the rat's investigation responses to the presentation of the Otest were significantly different in duration from that elicited by the Ohab during its fourth presentation (discrimination). The level of significance was set to 0.05.
Computational Modeling.
Our OB model has been extensively described elsewhere (35, 45). Briefly, it is a simplified representation of known OB circuitry constructed pursuant to the goal of determining the influence of NMDA-dependent synaptic plasticity on odor representations. Hence, it omits a number of established anatomical and pharmacological details that would not materially affect the outcome of the present simulations (46). The model includes four categories of neurons: olfactory sensory neurons, mitral cells, periglomerular cells, and granule cells, connected as depicted in Fig. 3A and detailed in its legend. All neurons were represented as single compartments except for mitral cells, which were represented by two compartments (the primary dendritic arborization and the soma with secondary dendrites). Each compartment was characterized by a membrane time constant that can be regarded as the mean product of the membrane capacitance and the membrane input resistance. Consequently, the evolution of the membrane voltage over time is described by a first-order differential equation:
where τ is the charging time constant of the neuron and Iext(t) is the total input at time t. The input from a particular presynaptic neuron at time t is computed as a function of the synaptic strength wij, the conductance change g(t) due to a presynaptic event xi(t0) at time t0, and the difference between the Nernst potential EN,ij of the associated channel type and the current membrane potential vj(t) of the postsynaptic neuron:
The time course of g(t) is described by a double exponential function:
All neurons in the model produced discrete spikes of unit amplitude for output, computed according to the instantaneous spiking probability, a continuous, bounded function of the membrane potential with a threshold θmin and a saturation value θmax.
In the simulations presented here, based on experimental evidence showing activity-dependent plasticity of these synapses (15–17), we varied the impact of OSNs onto mitral cell dendrites and of mitral cells onto granule cells in an activity-dependent manner. Synaptic strengths were first calculated from the parameters given in Table 2, which is published as supporting information on the PNAS web site, and responses to simulated odorants were obtained. These responses correspond to the preenrichment phase in the figures and in the behavioral experiments. To simulate synaptic plasticity due to daily exposure to a pair of odorants, the network was stimulated with two odorants and an activity-dependent plasticity rule was implemented to simulate potentiation of synaptic strength:
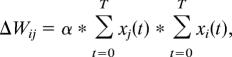 |
where Δwij is the change in synaptic strength associated with the synapse between neuron j and neuron i, and xi(t) and xj(t) are the outputs of neurons i and j at times t (x = 0 or x = 1).
Supplementary Material
Acknowledgments
This work was supported by Marie Curie Foundation Grant MOIF-CT-2005-51474 (to N.M.).
Abbreviations
- but
butanol
- dec
decanol
- dodec
dodecanol
- lim
limonene
- OB
olfactory bulb
- OSN
olfactory sensory neuron
- pent
pentanol
- terp
terpinen-4-ol
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Leggio M., Mandolesi L., Federico F., Spirito F., Ricci B., Gelfo F., Petrosini L. Behav. Brain Res. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Cancedda L., Putignano E., Sale A., Viegi A., Berardi N., Maffei L. J. Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rampon C., Tang Y., Goodhouse J., Shimizu E., Kyin M., Tsien J. Nat. Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 4.Bennett J., McRae P., Levy L., Frick K. Neurobiol. Learn. Mem. 2005;85:139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Kempermann G., Kuhn H., Gage F. J. Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham T., Soderstrom S., Winblad B., Mohammed A. Behav. Brain Res. 1999;103:63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 7.Frick K., Stearns N., Pan J., Berger-Sweeney J. Learn. Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummins R., Walsh R., Budtz-Olsen O., Konstantinos T., Horsfall C. Nature. 1973;243:516–518. doi: 10.1038/243516a0. [DOI] [PubMed] [Google Scholar]
- 9.Walsh T., Gandhi C., Stackman R. Behav. Neurosci. 1998;112:1114–1124. doi: 10.1037//0735-7044.112.5.1114. [DOI] [PubMed] [Google Scholar]
- 10.Morris R., Garrud P., Rawlins J., O’Keefe J. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 11.Kelche C., Roeser C., Jeltsch H., Cassel J., Will B. Neurobiol. Learn. Mem. 1995;63:155–166. doi: 10.1006/nlme.1995.1016. [DOI] [PubMed] [Google Scholar]
- 12.Mandairon N., Stack C., Kiselycznyk C., Linster C. Behav. Neurosci. 2006;120:173–179. doi: 10.1037/0735-7044.120.1.173. [DOI] [PubMed] [Google Scholar]
- 13.Linster C., Johnson B., Yue E., Morse A., Xu Z., Hingco E., Choi Y., Choi M., Messiha A., Leon M. J. Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linster C., Johnson B., Morse A., Yue E., Leon M. J. Neurosci. 2002;22:6842–6845. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ennis M., Linster C., Aroniadou-Anderjaska V., Ciombor K., Shipley M. T. Ann. N.Y. Acad. Sci. 1998;855:457–466. doi: 10.1111/j.1749-6632.1998.tb10606.x. [DOI] [PubMed] [Google Scholar]
- 16.Satou M., Hoshikawa R., Sato Y., Okawa K. J. Comp. Physiol. A. 2006;192:135–150. doi: 10.1007/s00359-005-0056-7. [DOI] [PubMed] [Google Scholar]
- 17.Satou M., Anzai S., Huruno M. J. Comp. Physiol. A. 2005;191:421–434. doi: 10.1007/s00359-005-0600-5. [DOI] [PubMed] [Google Scholar]
- 18.Kay L. Proc. Natl. Acad. Sci. USA. 2005;102:3863–3868. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Orive J., Mazor O., Turner G., Cassenaer S., Wilson R., Laurent G. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 20.Stopfer M., Bhagavan S., Smith B. H., Laurent G. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 21.Martin C., Gervais R., Hugues E., Messaoudi B., Ravel N. J. Neurosci. 2004;24:389–397. doi: 10.1523/JNEUROSCI.3433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleland T., Morse A., Yue E., Linster C. Behav. Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- 23.Linster C., Cleland T. A. J. Comput. Neurosci. 2001;10:187–193. doi: 10.1023/a:1011221131212. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson M., Perfilieva E., Johansson U., Orwar O., Eriksson P. J. Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 25.Kempermann G., Kuhn H., Gage F. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 26.Duffy S., Craddock K., Abel T., Nguyen P. Learn. Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franks K., Isaacson J. Neuron. 2005;47:101–114. doi: 10.1016/j.neuron.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Wilson D., Sullivan R., Leon M. Brain Res. 1985;354:314–317. doi: 10.1016/0165-3806(85)90186-5. [DOI] [PubMed] [Google Scholar]
- 29.Buonviso N., Chaput M. Neuroscience. 2000;95:325–332. doi: 10.1016/s0306-4522(99)00450-9. [DOI] [PubMed] [Google Scholar]
- 30.Buonviso N., Gervais R., Chalansonnet M., Chaput M. Eur. J. Neurosci. 1998;10:2472–2475. doi: 10.1046/j.1460-9568.1998.00266.x. [DOI] [PubMed] [Google Scholar]
- 31.Rochefort C., Gheusi G., Vincent J., Lledo P. J. Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban N., Sakmann B. J. Physiol. (London) 2002;542:355–367. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoi M., Mori K., Nakanishi S. Proc. Natl. Acad. Sci. USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margrie T., Sakmann B., Urban N. Proc. Natl. Acad. Sci. USA. 2001;98:319–324. doi: 10.1073/pnas.011523098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linster C., Cleland T. A. J. Comput. Neurosci. 2004;16:39–47. doi: 10.1023/b:jcns.0000004840.87570.2e. [DOI] [PubMed] [Google Scholar]
- 36.Urban N. Physiol. Behav. 2002;77:607–612. doi: 10.1016/s0031-9384(02)00895-8. [DOI] [PubMed] [Google Scholar]
- 37.Laurent G. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- 38.Mori K., Nagao H., Yoshihara Y. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher M., Wilson D. J. Neurosci. 2003;23:6946–6955. doi: 10.1523/JNEUROSCI.23-17-06946.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isaacson J., Strowbridge B. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 41.Wilson D. Brain Res. 1995;677:238–242. doi: 10.1016/0006-8993(95)00151-f. [DOI] [PubMed] [Google Scholar]
- 42.Staubli U., Thibault O., DiLorenzo M., Lynch G. Behav. Neurosci. 1989;103:54–60. doi: 10.1037//0735-7044.103.1.54. [DOI] [PubMed] [Google Scholar]
- 43.Wiltrout C., Dogra S., Linster C. Behav. Neurosci. 2003;117:236–245. doi: 10.1037/0735-7044.117.2.236. [DOI] [PubMed] [Google Scholar]
- 44.Yue E., Cleland T., Pavlis M., Linster C. Behav. Neurosci. 2004;118:184–190. doi: 10.1037/0735-7044.118.1.184. [DOI] [PubMed] [Google Scholar]
- 45.Linster C., Cleland T. Neural Networks. 2002;15:709–717. doi: 10.1016/s0893-6080(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 46.Ennis M., Zimmer L. A., Shipley M. T. NeuroReport. 1996;7:989–992. doi: 10.1097/00001756-199604100-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.