Abstract
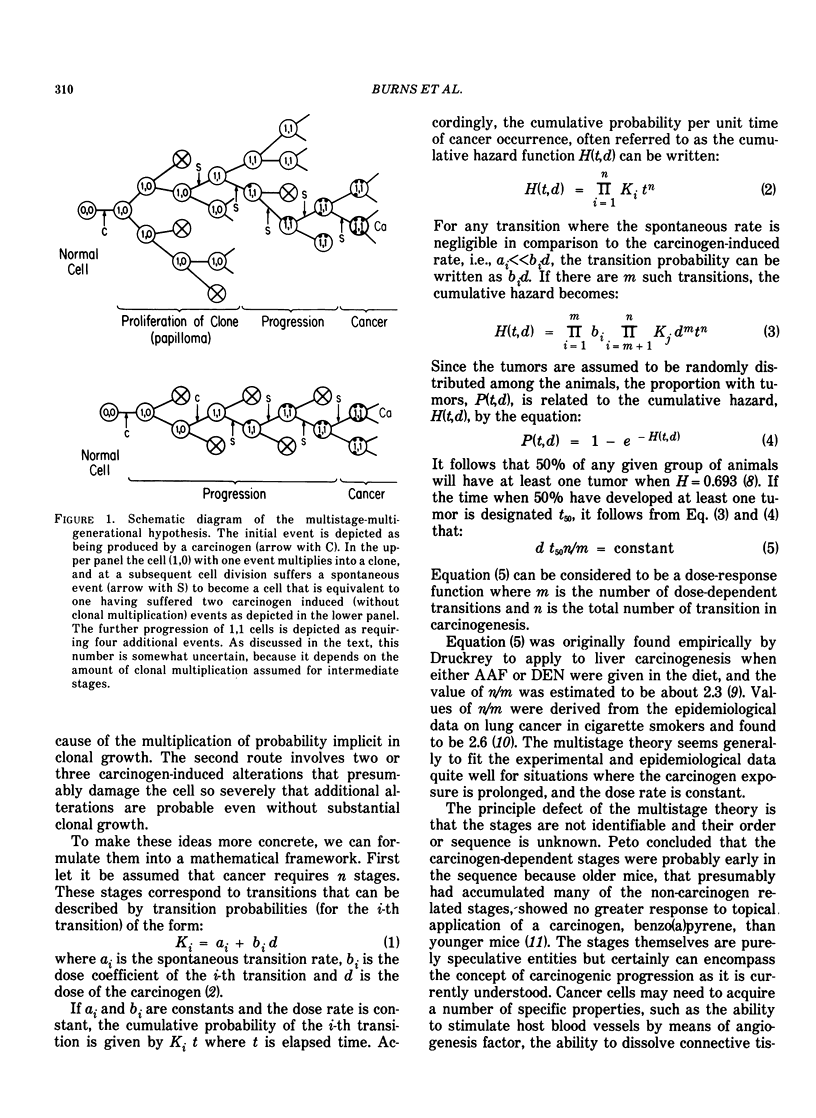
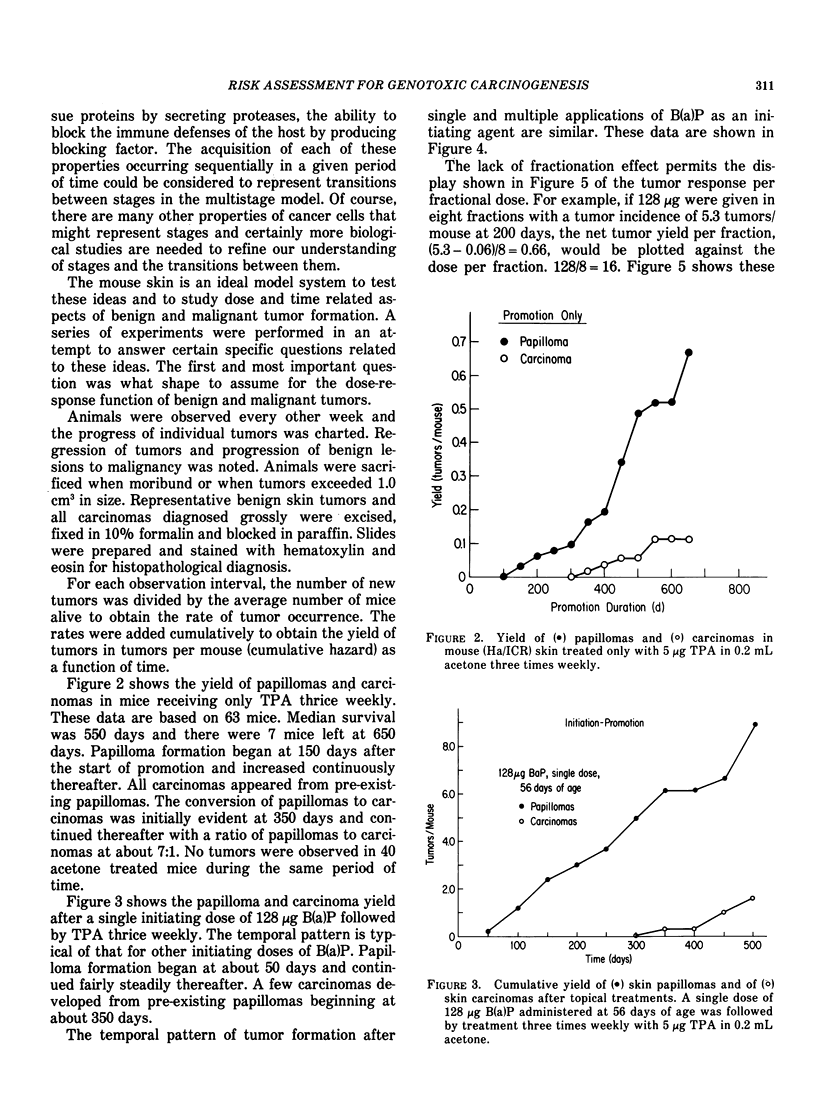
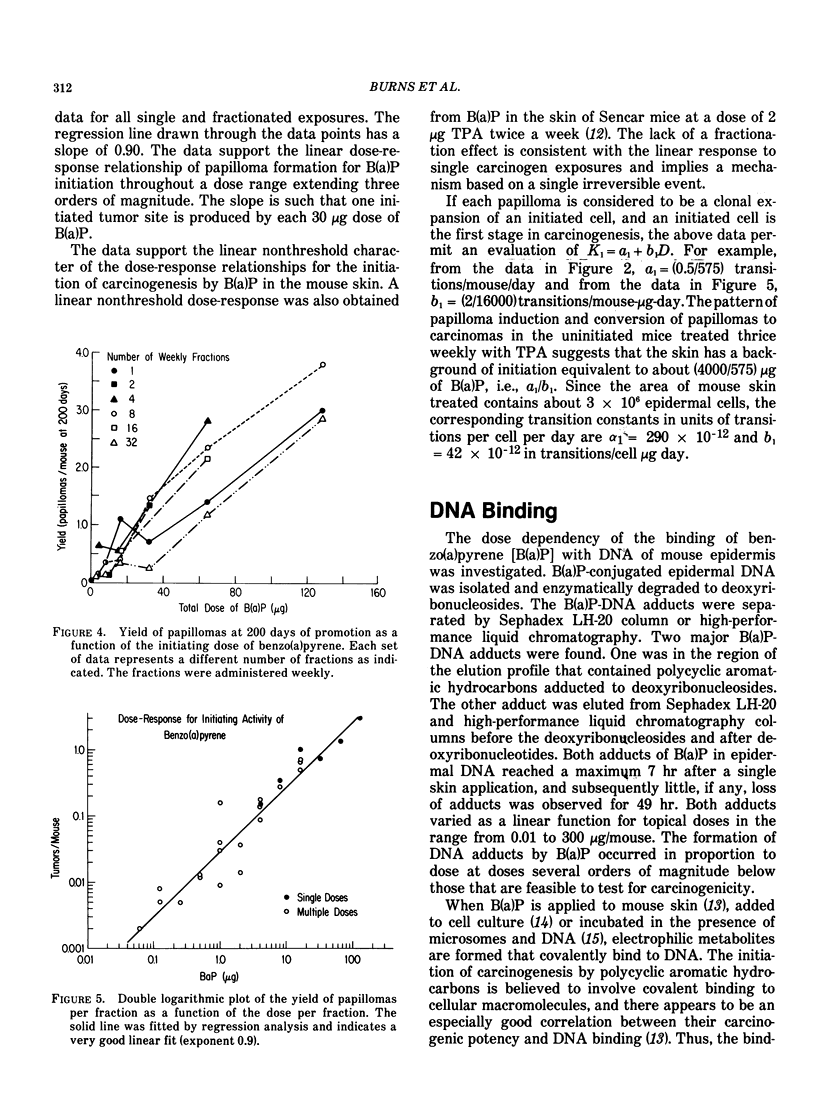
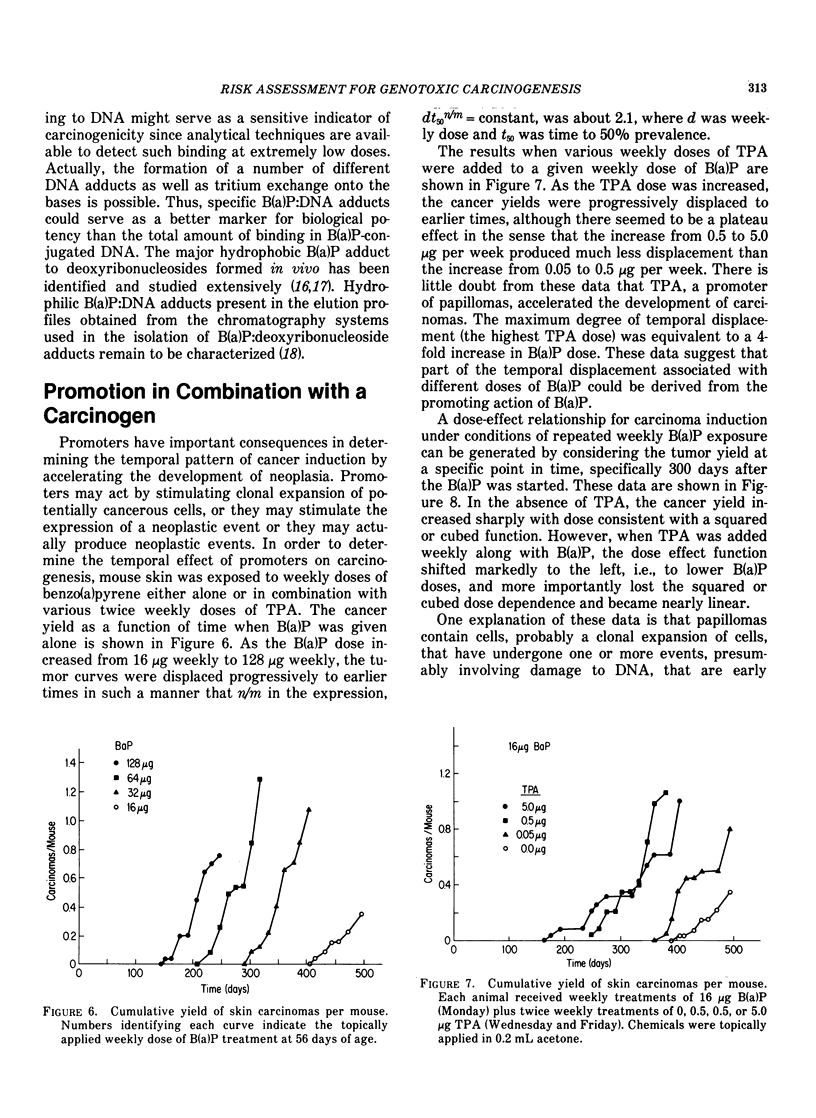
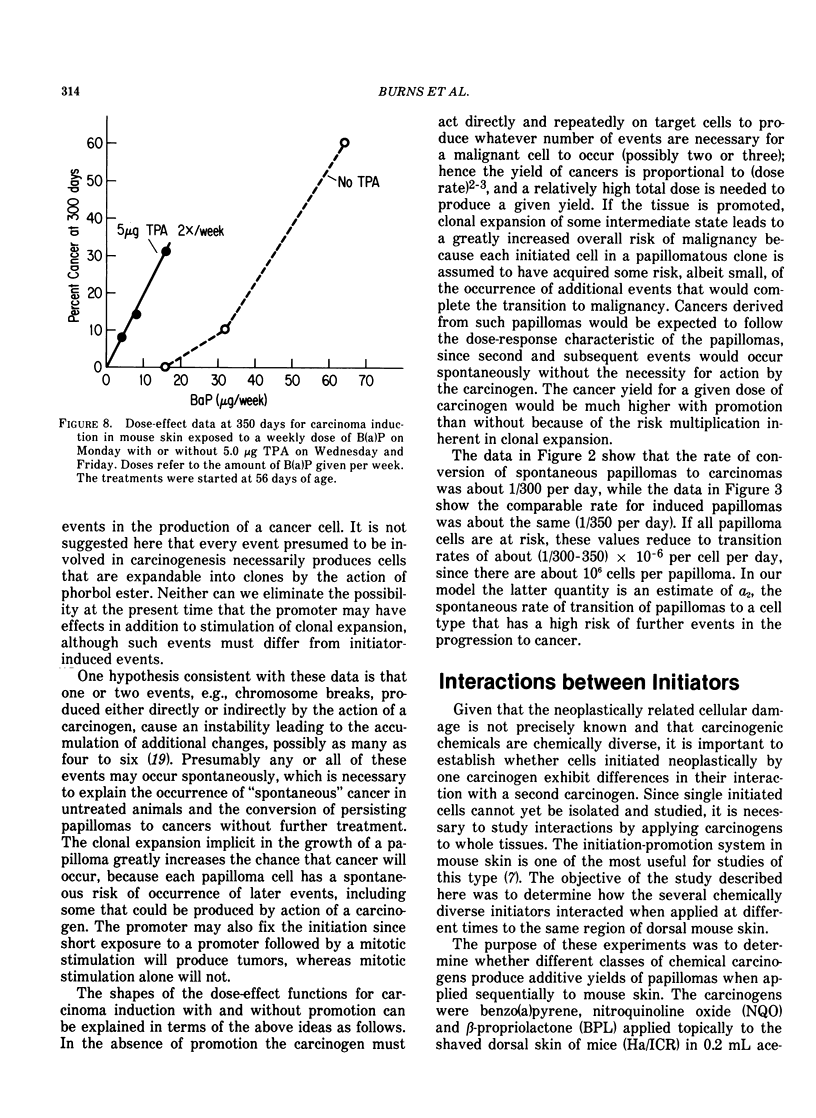
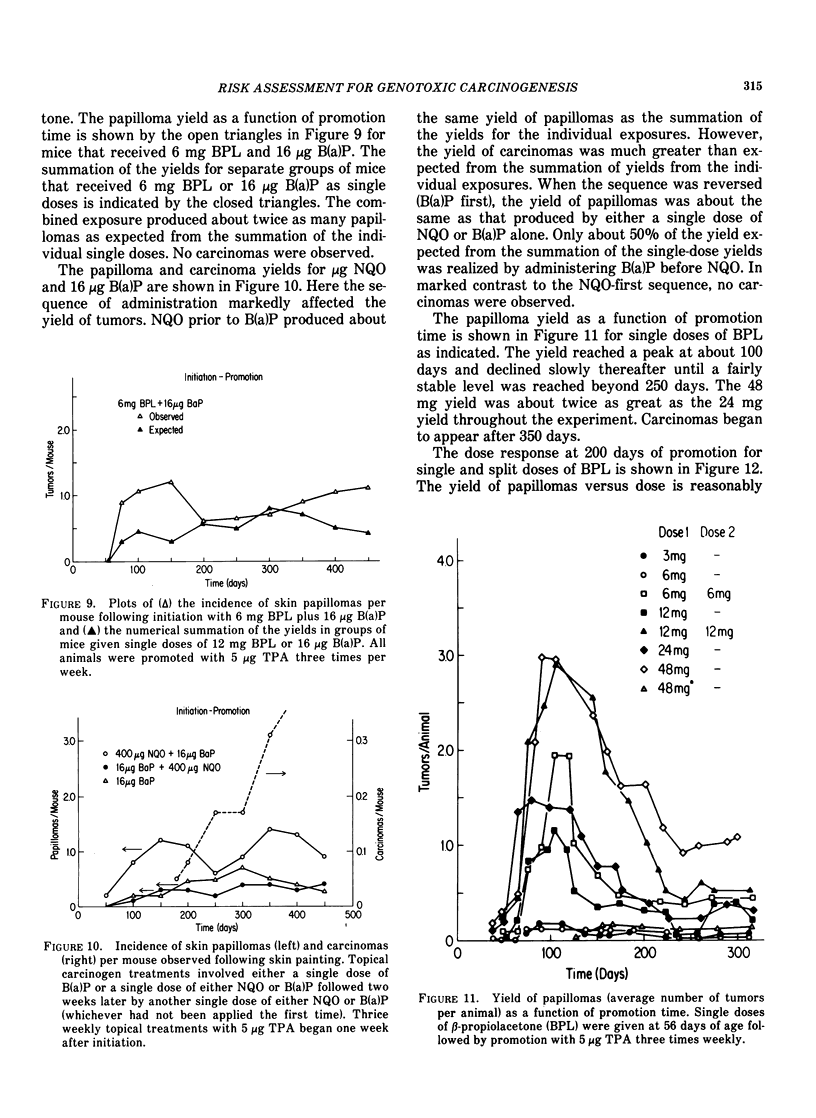
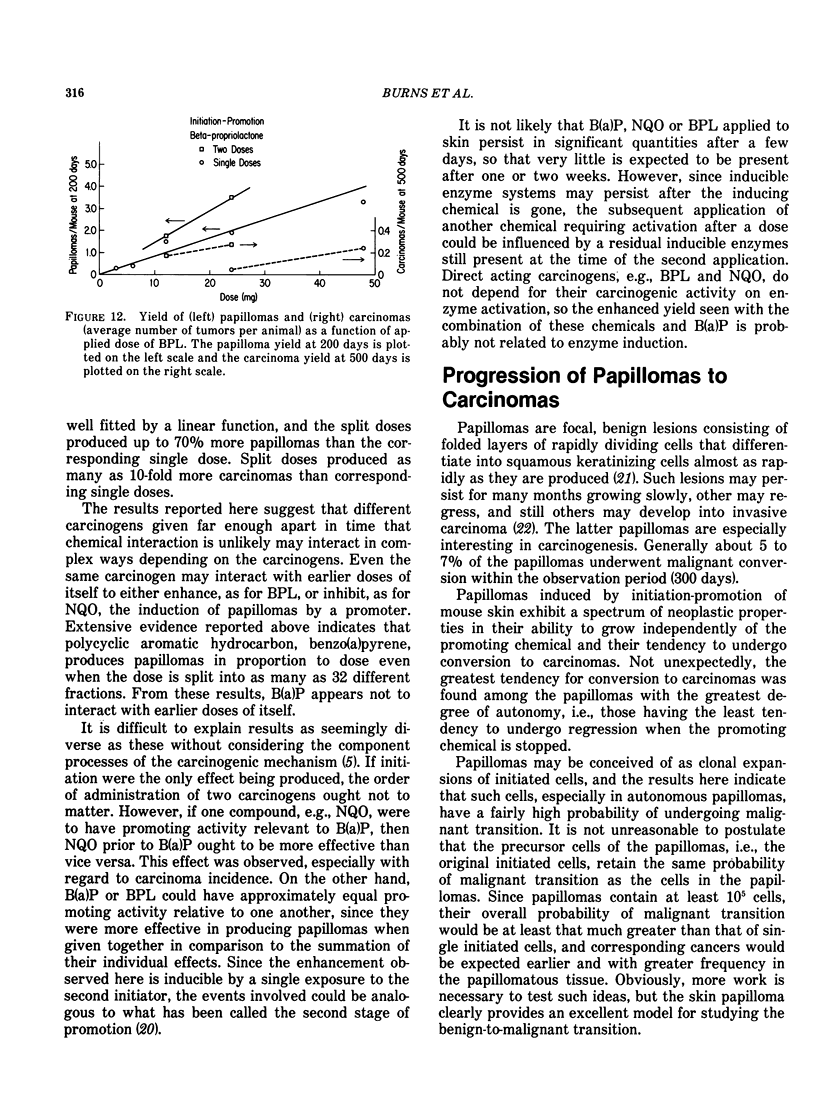
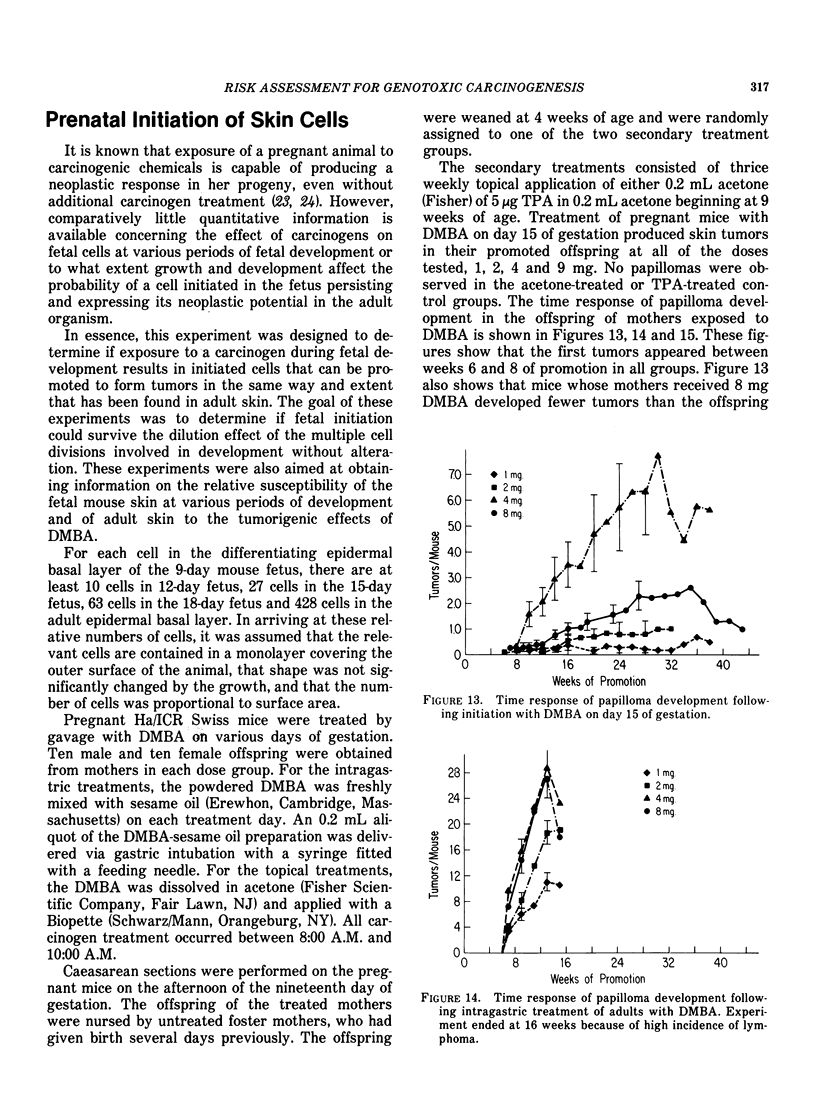
Tumor induction data in the mouse skin initiation-promotion system were found to be consistent with a quadratic function where the coefficient of the linear term depended on the dose of the promoter. The model implies that the existence of promoters may be more important at low doses of the carcinogen than at high doses where most testing is performed. Experiments are described showing that the initiating effect of carcinogenic chemicals, such as benzo(a)pyrene, 7,12-dimethyl-benz(a)anthracene, nitroquinoline oxide and beta-propiolactone, accumulates in a linear, irreversible manner at low doses. Even when 7,12-dimethylbenz(a)anthracene was applied intragastrically to pregnant females, initiating activity was found in the skins of exposed offspring about in proportion to dose applied and number of cells at risk. The initiated cells essentially represent a potential for cancer that has a high probability for expression in the presence of a promoter. Risk then can be interpreted in terms of the accumulated dose of initiator which alone presents a small risk of cancer. However, a promoter may substantially expand the overall risk, possibly by clonally expanding the initiated cells. Promotion needs to be sustained since there is a reduction of cancer risk if promotion is ended early. Some tissues, such as mouse bladder, may be intrinsically promoted more than others so that comparisons between tissues and between species are best made when the combination of intrinsic promotion and response to extrinsic promotion are comparable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMITAGE P., DOLL R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 1954 Mar;8(1):1–12. doi: 10.1038/bjc.1954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EVIDENCE FOR THE BINDING OF POLYNUCLEAR AROMATIC HYDROCARBONS TO THE NUCLEIC ACIDS OF MOUSE SKIN: RELATION BETWEEN CARCINOGENIC POWER OF HYDROCARBONS AND THEIR BINDING TO DEOXYRIBONUCLEIC ACID. Nature. 1964 May 23;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- Burns F. J., Vanderlaan M., Sivak A., Albert R. E. Regression kinetics of mouse skin papillomas. Cancer Res. 1976 Apr;36(4):1422–1427. [PubMed] [Google Scholar]
- Crump K. S., Hoel D. G., Langley C. H., Peto R. Fundamental carcinogenic processes and their implications for low dose risk assessment. Cancer Res. 1976 Sep;36(9 PT1):2973–2979. [PubMed] [Google Scholar]
- Duncan M., Brookes P., Dipple A. Metabolism and binding to cellular macromolecules of a series of hydrocarbons by mouse embryo cells in culture. Int J Cancer. 1969 Nov 15;4(6):813–819. doi: 10.1002/ijc.2910040610. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V. A microsome-dependent binding of benzo[a]pyrene to DNA. Cancer Res. 1969 Jun;29(6):1272–1276. [PubMed] [Google Scholar]
- Grover P. L., Hewer A., Pal K., Sims P. The involvement of a diol-epoxide in the metabolic activation of benzo(a)pyrene in human bronchial mucosa and in mouse skin. Int J Cancer. 1976 Jul 15;18(1):1–6. doi: 10.1002/ijc.2910180102. [DOI] [PubMed] [Google Scholar]
- Iannaccone P. M., Gardner R. L., Harris H. The cellular origin of chemically induced tumours. J Cell Sci. 1978 Feb;29:249–269. doi: 10.1242/jcs.29.1.249. [DOI] [PubMed] [Google Scholar]
- Meehan T., Straub K., Calvin M. Benzo[alpha]pyrene diol epoxide covalently binds to deoxyguanosine and deoxyadenosine in DNA. Nature. 1977 Oct 20;269(5630):725–727. doi: 10.1038/269725a0. [DOI] [PubMed] [Google Scholar]
- Peto R., Roe F. J., Lee P. N., Levy L., Clack J. Cancer and ageing in mice and men. Br J Cancer. 1975 Oct;32(4):411–426. doi: 10.1038/bjc.1975.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Bowden G. T., Scribner J. D., Boutwell R. K. Dose-response studies on the ability of 7,12-dimethylbenz(alpha)anthracene and benz(alpha)anthracene to initiate skin tumors. J Natl Cancer Inst. 1974 Nov;53(5):1337–1340. doi: 10.1093/jnci/53.5.1337. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M., Nelson K., Gleason G. L. Studies on the mechanism of skin tumor promotion: evidence for several stages in promotion. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3659–3663. doi: 10.1073/pnas.77.6.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesselinovitch S. D., Rao K. V., Mihailovich N. Neoplastic response of mouse tissues during perinatal age periods and its significance in chemical carcinogenesis. Natl Cancer Inst Monogr. 1979 May;(51):239–250. [PubMed] [Google Scholar]
- Weinstein I. B., Jeffrey A. M., Jennette K. W., Blobstein S. H., Harvey R. G., Harris C., Autrup H., Kasai H., Nakanishi K. Benzo(a)pyrene diol epoxides as intermediates in nucleic acid binding in vitro and in vivo. Science. 1976 Aug 13;193(4253):592–595. doi: 10.1126/science.959820. [DOI] [PubMed] [Google Scholar]


