Abstract
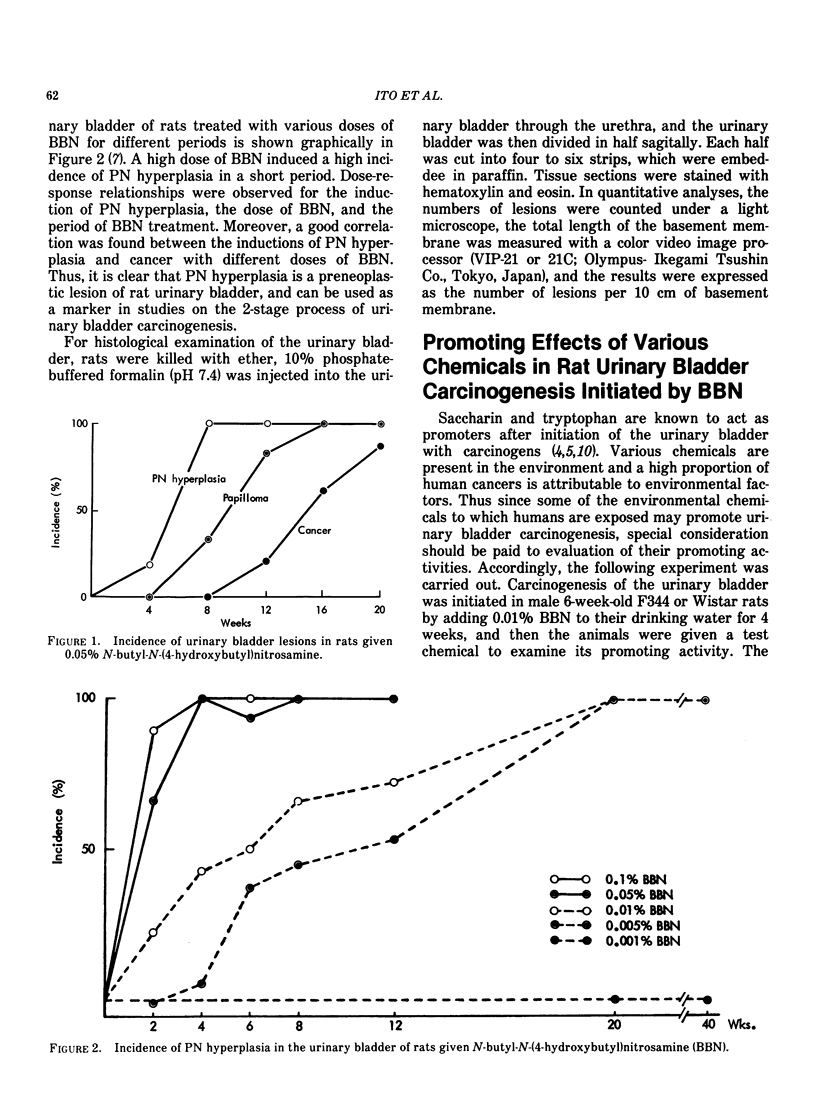
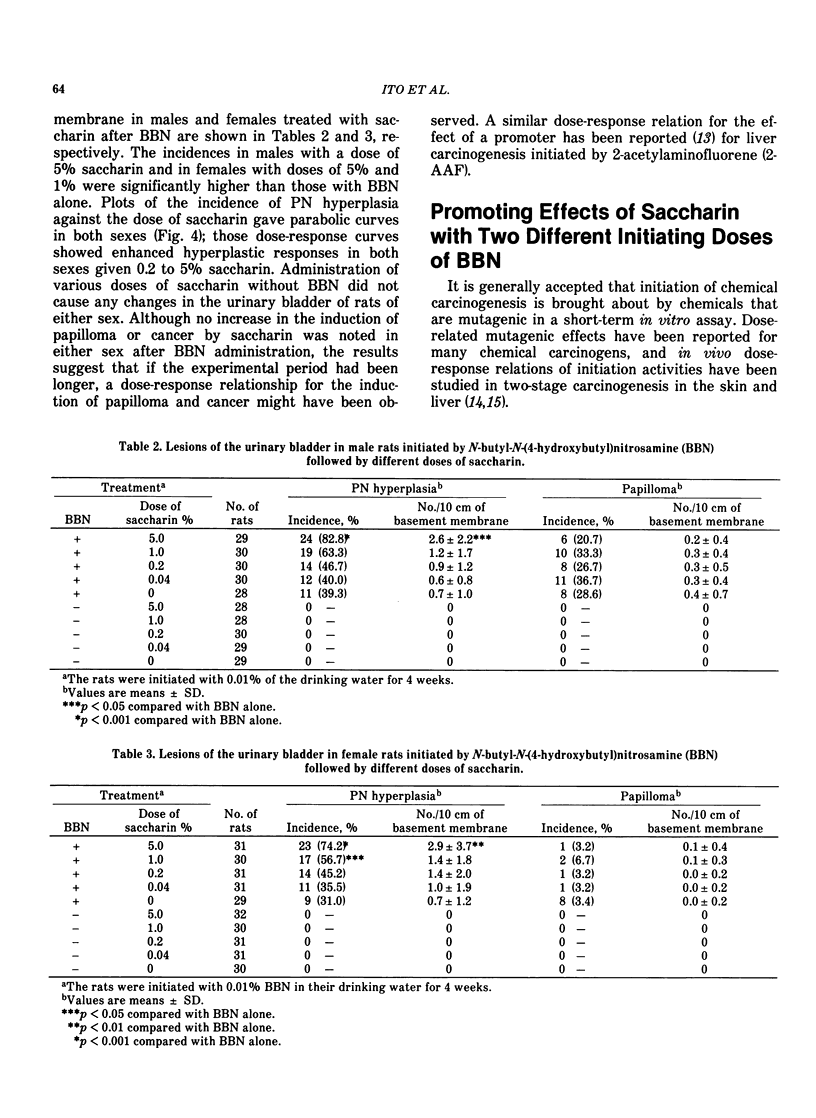
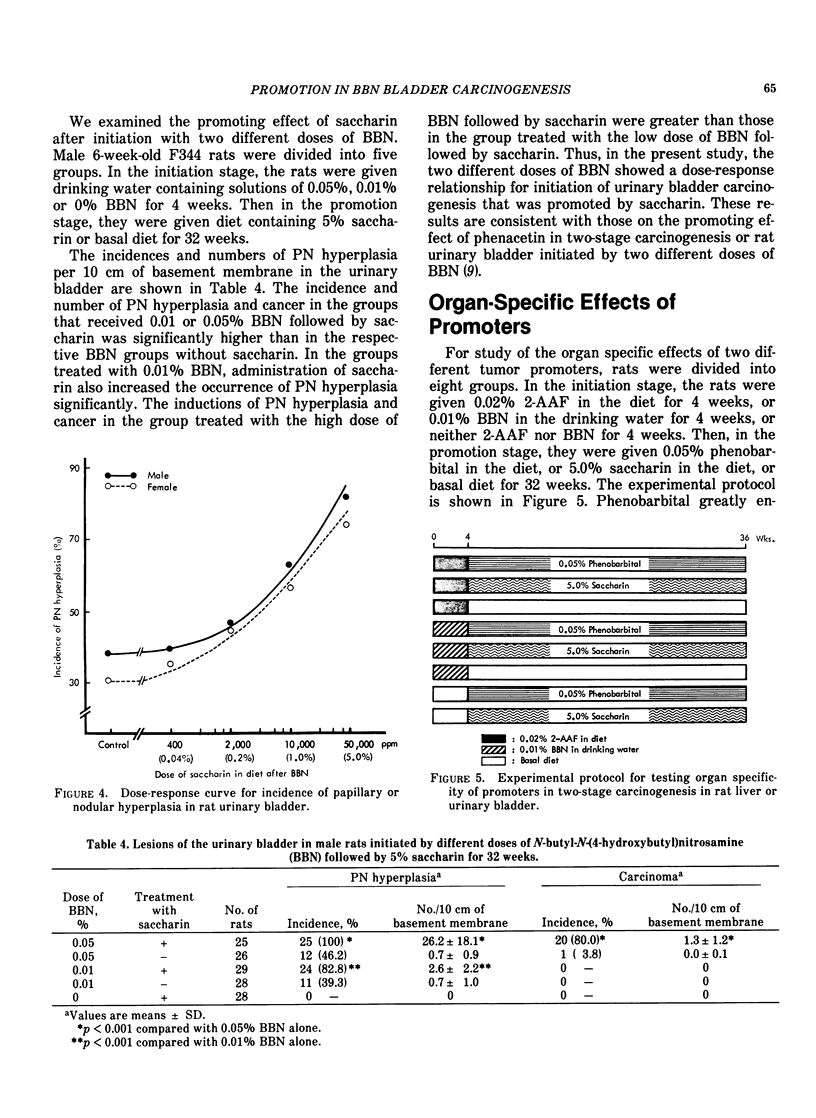
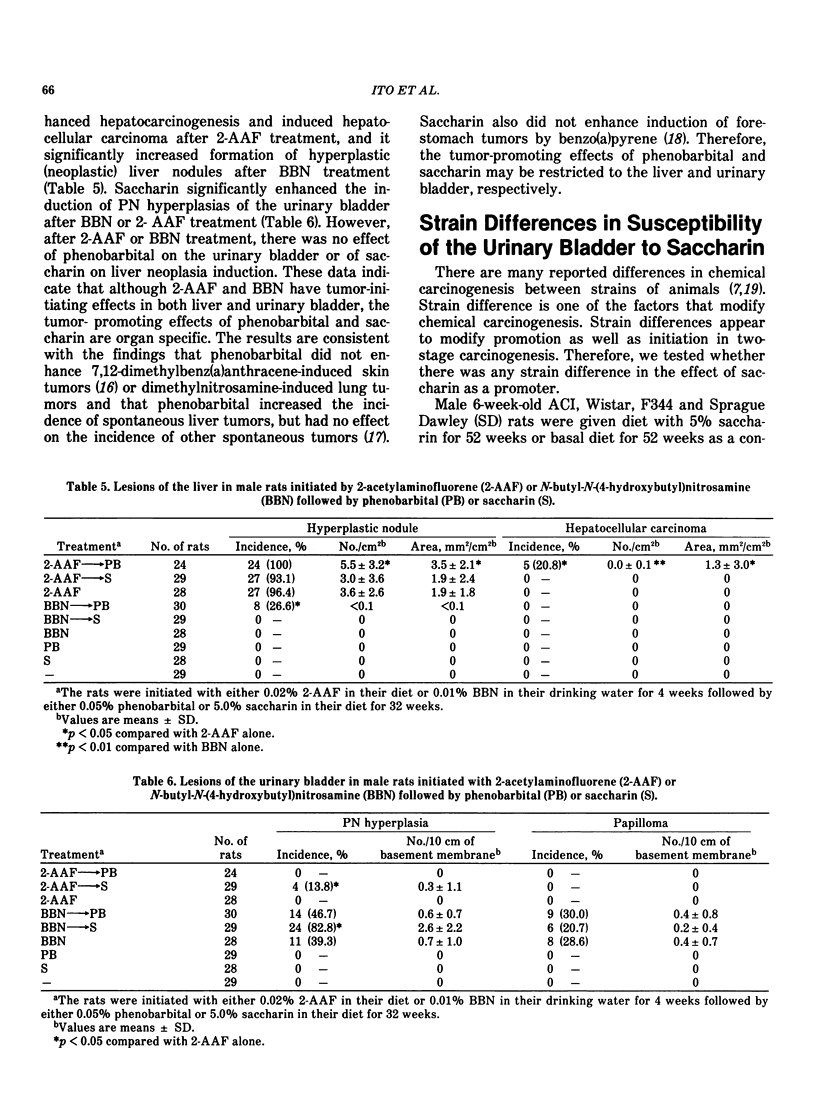
It has been shown that the occurrence of the preneoplastic lesion, papillary or nodular hyperplasia (PN hyperplasia) in rat urinary bladder induced by carcinogens is correlated with that of cancer. Therefore, the promoting effects of chemicals in two-stage bladder carcinogenesis were judged by measuring their ability to induce PN hyperplasia in rats. Male rats were given N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN) for 4 weeks and then one of 16 test chemicals for 32 to 34 weeks. Saccharin, ascorbate, DL-tryptophan, allopurinol, and diphenyl promoted development of PN hyperplasia. The dose-response of the promoters were examined in both sexes of rats by administration of saccharin at doses of 0.04, 0.2, 1.0 and 5.0% for 32 weeks after BBN treatment. The occurrence of PN hyperplasia was significantly increased in the group given 5% saccharin. Dose-response curves showed enhanced hyperplastic responses in both sexes given 0.2 to 5% saccharin. The organ specificities of promoters were studied in rats initiated with BBN or 2-acetylamino-fluorene (2-AAF) followed by phenobarbital or saccharin for 32 weeks. Phenobarbital greatly enhanced hepatocarcinogenesis. Saccharin significantly enhanced the occurrence of both BBN-induced and 2-AAF-induced PN hyperplasia. However, there was no effect of phenobarbital on the urinary bladder or of saccharin on the liver. The rats showed a strain difference in susceptibility of the urinary bladder to saccharin; ACI rats were most susceptible and Sprague Dawley rats were most resistant to saccharin. The membrane potential of superficial epithelial cells in the urinary bladder of rats treated with saccharin was measured with an intracellular microelectrode and found to be higher than that of controls.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai M., Kani T., Sugihara S., Matsumura K., Miyata Y., Shinohara Y., Ito N. Scanning and transmission electron microscopy of changes in the urinary bladder in rats treated with N-butyl-N-(4-hydroxybutyl)nitrosoamine. Gan. 1974 Dec;65(6):529–540. [PubMed] [Google Scholar]
- Cohen S. M., Arai M., Jacobs J. B., Friedell G. H. Promoting effect of saccharin and DL-tryptophan in urinary bladder carcinogenesis. Cancer Res. 1979 Apr;39(4):1207–1217. [PubMed] [Google Scholar]
- Fukushima S., Cohen S. M. Saccharin-induced hyperplasia of the rat urinary bladder. Cancer Res. 1980 Mar;40(3):734–736. [PubMed] [Google Scholar]
- Fukushima S., Friedell G. H., Jacobs J. B., Cohen S. M. Effect of L-tryptophan and sodium saccharin on urinary tract carcinogenesis initiated by N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide. Cancer Res. 1981 Aug;41(8):3100–3103. [PubMed] [Google Scholar]
- Grube D. D., Peraino C., Fry R. J. The effect ofdietary phenobarbital on the induction of skin tumors in hairless mice with 7, 12-dimethylbenz(a)anthracene. J Invest Dermatol. 1975 Apr;64(4):258–262. doi: 10.1111/1523-1747.ep12510676. [DOI] [PubMed] [Google Scholar]
- Hicks R. M., Wakefield J., Chowaniec J. Evaluation of a new model to detect bladder carcinogens or co-carcinogens; results obtained with saccharin, cyclamate and cyclophosphamide. Chem Biol Interact. 1975 Sep;11(3):225–233. doi: 10.1016/0009-2797(75)90101-5. [DOI] [PubMed] [Google Scholar]
- Ito N., Hiasa Y., Tamai A., Okajima E., Kitamura H. Histogenesis of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in rats. Gan. 1969 Aug;60(4):401–410. [PubMed] [Google Scholar]
- Ito N., Matayoshi K., Arai M., Yoshioka Y., Kamamoto Y. Effect of various factors on induction of urinary bladder tumors in animals by N-butyl-n-(4-hydroxybutyl)nitrosoamine. Gan. 1973 Apr;64(2):151–159. [PubMed] [Google Scholar]
- Jacobs J. B., Arai M., Cohen S. M., Friedell G. H. Early lesions in experimental bladder cancer: scanning electron microscopy of cell surface markers. Cancer Res. 1976 Jul;36(7 Pt 2):2512–2517. [PubMed] [Google Scholar]
- Kakizoe T., Kawachi T., Okada M. Concanavalin A agglutination of bladder cells of rats treated with bladder carcinogens; a rapid new test to detect bladder carcinogens. Cancer Lett. 1978 Nov;5(5):285–290. doi: 10.1016/s0304-3835(78)80026-3. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Fukushima S., Shibata M., Shirai T., Ogiso T., Ito N. Effect of phenacetin and caffeine on the urinary bladder of rats treated with N-butyl-N-(4-hydroxybutyl)nitrosamine. Gan. 1978 Jun;69(3):395–400. [PubMed] [Google Scholar]
- Nakanishi K., Hagiwara A., Shibata M., Imaida K., Tatematsu M., Ito N. Dose response of saccharin in induction of urinary bladder hyperplasias in Fischer 344 rats pretreated with N-butyl-N-(4-hydroxybutyl)nitrosamine. J Natl Cancer Inst. 1980 Nov;65(5):1005–1010. [PubMed] [Google Scholar]
- Narisawa T., Magadia N. E., Weisburger J. H., Wynder E. L. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974 Oct;53(4):1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- Peraino C., Fry R. J., Staffeldt E. Reduction and enhancement by phenobarbital of hepatocarcinogenesis induced in the rat by 2-acetylaminofluorene. Cancer Res. 1971 Oct;31(10):1506–1512. [PubMed] [Google Scholar]
- Peraino C., Staffeldt E. F., Haugen D. A., Lombard L. S., Stevens F. J., Fry R. J. Effects of varying the dietary concentration of phenobarbital on its enhancement of 2-acetylaminofluorene-induced hepatic tumorigenesis. Cancer Res. 1980 Sep;40(9):3268–3273. [PubMed] [Google Scholar]
- Preussmann R. Dose-response studies and 'no-effect-levels' of N-nitroso compounds: some general aspects. Oncology. 1980;37(4):243–250. doi: 10.1159/000225445. [DOI] [PubMed] [Google Scholar]
- Roe F. J., Levy L. S., Carter R. L. Feeding studies on sodium cyclamate, saccharin and sucrose for carcinogenic and tumour-promoting activity. Food Cosmet Toxicol. 1970 Apr;8(2):135–145. doi: 10.1016/s0015-6264(70)80332-7. [DOI] [PubMed] [Google Scholar]
- Shinohara Y., Ogiso T., Hananouchi M., Nakanishi K., Yoshimura T., Ito N. Effect of various factors on the induction of liver tumors in animals by quinoline. Gan. 1977 Dec;68(6):785–796. [PubMed] [Google Scholar]
- Slaga T. J., Bowden G. T., Scribner J. D., Boutwell R. K. Dose-response studies on the ability of 7,12-dimethylbenz(alpha)anthracene and benz(alpha)anthracene to initiate skin tumors. J Natl Cancer Inst. 1974 Nov;53(5):1337–1340. doi: 10.1093/jnci/53.5.1337. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Lee G., Farber E. Induction of resistant hepatocytes as a new principle for a possible short-term in vivo test for carcinogens. Cancer Res. 1980 Apr;40(4):1157–1164. [PubMed] [Google Scholar]


